Substance Formed By The Chemical Combination Of Elements
Muz Play
Mar 15, 2025 · 6 min read
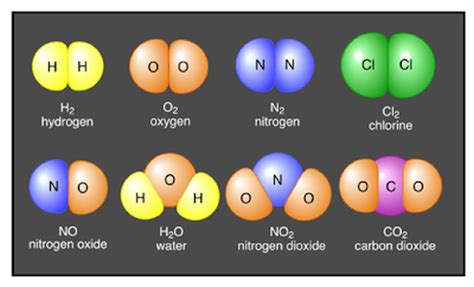
Table of Contents
Substances Formed by the Chemical Combination of Elements: A Deep Dive into Compounds and Molecules
The world around us is a tapestry woven from the intricate interactions of elements. These fundamental building blocks, listed in the periodic table, rarely exist in isolation. Instead, they combine through chemical reactions to form an astonishing array of substances, creating the diverse materials we encounter daily – from the air we breathe to the food we eat, and the devices we use. This article will delve into the fascinating realm of substances formed through the chemical combination of elements, exploring the concepts of compounds, molecules, their properties, and the forces that govern their formation.
Understanding Chemical Bonds: The Glue That Holds It Together
Before diving into the specifics of different substances, it’s crucial to understand the fundamental forces that bind elements together: chemical bonds. These bonds arise from the electrostatic interactions between atoms, primarily involving their outermost electrons, known as valence electrons. The desire for atoms to achieve a stable electron configuration, often resembling that of a noble gas (a full outer electron shell), drives the formation of chemical bonds. Three major types of chemical bonds dominate the formation of substances:
1. Ionic Bonds: The Electrostatic Attraction
Ionic bonds form when one or more electrons are transferred from one atom to another. This transfer creates ions: positively charged cations (atoms that have lost electrons) and negatively charged anions (atoms that have gained electrons). The resulting electrostatic attraction between these oppositely charged ions holds them together, forming an ionic compound. A classic example is sodium chloride (NaCl), or common table salt. Sodium (Na) readily loses one electron to become a Na⁺ cation, while chlorine (Cl) readily gains one electron to become a Cl⁻ anion. The strong electrostatic attraction between these ions results in the formation of a crystalline structure.
Key characteristics of ionic compounds:
- High melting and boiling points due to strong electrostatic attractions.
- Usually soluble in water.
- Conduct electricity when dissolved in water or molten.
- Often brittle and crystalline in structure.
2. Covalent Bonds: Sharing is Caring
Covalent bonds involve the sharing of one or more pairs of electrons between atoms. This sharing allows each atom to achieve a more stable electron configuration. Covalent bonds are prevalent in many organic compounds and molecules. For example, in a water molecule (H₂O), each hydrogen atom shares one electron with the oxygen atom, forming a stable molecule. The strength of a covalent bond depends on the number of shared electron pairs and the electronegativity of the atoms involved.
Key characteristics of covalent compounds:
- Lower melting and boiling points compared to ionic compounds, varying widely depending on the molecule's size and polarity.
- Solubility in water varies greatly, depending on the polarity of the molecule.
- Generally poor conductors of electricity.
- Can exist as gases, liquids, or solids at room temperature.
3. Metallic Bonds: A Sea of Electrons
Metallic bonds occur in metals. In a metal, valence electrons are delocalized, meaning they are not associated with any particular atom but rather move freely throughout the metal lattice. This "sea" of electrons accounts for many of the characteristic properties of metals.
Key characteristics of metallic compounds:
- High melting and boiling points, varying depending on the metal.
- Excellent conductors of heat and electricity.
- Malleable (can be hammered into shapes) and ductile (can be drawn into wires).
- Often lustrous (shiny).
Compounds: A Deeper Look into Chemical Combinations
A compound is a pure substance formed when two or more different elements combine chemically in a fixed ratio. This ratio is determined by the atoms' valency – their combining capacity. The properties of a compound are entirely different from the properties of its constituent elements. For instance, sodium (a highly reactive metal) and chlorine (a poisonous gas) combine to form sodium chloride (table salt), a harmless and essential component of our diet. This highlights the transformative power of chemical bonding. Compounds can be classified based on their chemical composition and properties. Some common categories include:
Types of Compounds
- Oxides: Compounds containing oxygen and one other element. Examples include water (H₂O), carbon dioxide (CO₂), and iron oxide (Fe₂O₃).
- Acids: Compounds that release hydrogen ions (H⁺) when dissolved in water. Examples include hydrochloric acid (HCl) and sulfuric acid (H₂SO₄).
- Bases: Compounds that release hydroxide ions (OH⁻) when dissolved in water. Examples include sodium hydroxide (NaOH) and calcium hydroxide (Ca(OH)₂).
- Salts: Compounds formed from the reaction of an acid and a base. Table salt (NaCl) is a classic example.
- Organic Compounds: Compounds containing carbon, usually bonded to hydrogen, oxygen, nitrogen, and other elements. This vast class encompasses a huge variety of molecules, including carbohydrates, lipids, proteins, and nucleic acids – the building blocks of life.
Molecules: The Discrete Units of Matter
A molecule is a group of two or more atoms held together by covalent bonds. It represents the smallest unit of a substance that retains the chemical properties of that substance. While all molecules are compounds, not all compounds are molecules. Ionic compounds, for instance, exist as a lattice of ions rather than discrete molecules. The properties of molecules are dictated by their structure, including the types of atoms present, the arrangement of atoms, and the types of bonds connecting them. The concept of molecular geometry is crucial in understanding molecular properties, including polarity and reactivity.
Molecular Geometry and Properties
The three-dimensional arrangement of atoms within a molecule significantly influences its properties. For example, the bent shape of a water molecule leads to its polarity, making it an excellent solvent for many ionic and polar substances. Conversely, the linear shape of carbon dioxide results in a non-polar molecule. Understanding molecular geometry is critical in predicting the behavior of molecules in various contexts.
Chemical Formulas and Nomenclature: Describing Substances
Chemical formulas provide a concise way to represent the composition of compounds and molecules. They indicate the types of atoms present and their relative numbers. For example, the formula H₂O indicates that a water molecule contains two hydrogen atoms and one oxygen atom. The systematic naming of compounds, known as chemical nomenclature, follows established rules and conventions to ensure clear and unambiguous communication among scientists.
The Importance of Studying Substances and their Formation
The study of substances and their formation is fundamental to numerous fields, including:
- Chemistry: Understanding chemical reactions and the properties of substances is the cornerstone of chemistry.
- Materials Science: The development of new materials with specific properties relies heavily on the knowledge of chemical bonding and the properties of compounds.
- Biology: Living organisms are composed of a vast array of substances, and their interactions drive biological processes.
- Medicine: Many pharmaceuticals are compounds carefully designed to interact with specific biological targets.
- Environmental Science: Understanding the chemical composition of the environment is crucial for monitoring pollution and developing remediation strategies.
Conclusion: A World Built on Chemical Combinations
The chemical combination of elements is the fundamental process that shapes our world. From the simplest molecules to the most complex compounds, the intricate interplay of atoms and the forces that bind them generates the immense diversity of substances we observe. By understanding the principles of chemical bonding and the properties of compounds and molecules, we gain insights into the underlying mechanisms that govern the natural world and empower us to create new materials and technologies. The ongoing exploration of this fascinating field promises to reveal even more remarkable insights into the structure and behavior of matter.
Latest Posts
Latest Posts
-
How Many Protons Does Iodine Have
Mar 15, 2025
-
If The Finches On The Galapagos Islands
Mar 15, 2025
-
How To Find A Perpendicular Vector
Mar 15, 2025
-
How Could Sulfur Form An Ion
Mar 15, 2025
-
What Elemsnts Are Most Likey To Turn Into Anions Why
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Substance Formed By The Chemical Combination Of Elements . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
