The Arrangement Of The Periodic Table
Muz Play
Mar 25, 2025 · 6 min read
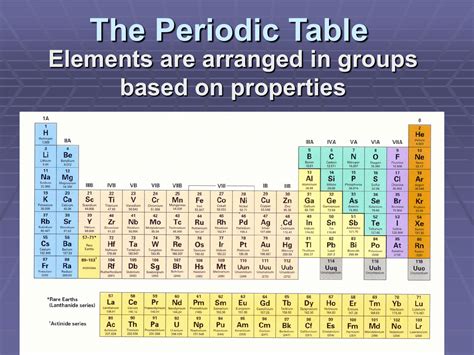
Table of Contents
The Arrangement of the Periodic Table: A Deep Dive into the Organization of Elements
The periodic table, a cornerstone of chemistry, isn't just a random collection of elements. Its meticulously organized structure reflects fundamental properties and relationships between atoms, providing a powerful tool for predicting chemical behavior and understanding the building blocks of matter. This comprehensive guide delves into the arrangement of the periodic table, exploring its history, underlying principles, and the significance of its various features.
A Brief History: From Chaos to Order
Before the elegant organization we know today, elements existed as a scattered collection of observations. Alchemists, in their pursuit of transmutation, amassed a growing list of substances. However, it was Dmitri Mendeleev in 1869 who achieved the breakthrough. Recognizing patterns in atomic weights and chemical properties, he arranged the then-known elements in order of increasing atomic weight, noticing recurring trends. He ingeniously left gaps for yet-to-be-discovered elements, predicting their properties based on the periodic pattern – a testament to the power of his system. While Mendeleev's original table was based on atomic weight, the modern periodic table is organized by atomic number, reflecting the number of protons in an atom's nucleus. This crucial change solidified the table's predictive capabilities and corrected some anomalies in Mendeleev's original arrangement.
The Fundamentals: Rows, Columns, and Blocks
The periodic table's structure is characterized by its rows (periods) and columns (groups). Each row represents an energy level, with elements in the same row having the same number of electron shells. Moving across a period, electrons are added progressively to the outermost shell, leading to a gradual change in properties. The columns, on the other hand, group elements with similar chemical properties because they possess the same number of valence electrons – the electrons in the outermost shell that participate in chemical bonding.
Periods: Energy Levels and Trends
The seven periods reflect the seven principal electron shells. Each period starts with an alkali metal (Group 1) and generally ends with a noble gas (Group 18). The length of the period varies, reflecting the filling of subshells (s, p, d, and f). The first period is exceptionally short, containing only hydrogen and helium, as it represents the filling of the 1s subshell. The second and third periods are longer, incorporating the filling of the 2s and 2p, and 3s and 3p subshells, respectively. The subsequent periods become increasingly longer, accommodating the filling of the d and f subshells.
Groups: Valence Electrons and Chemical Behavior
Elements within the same group share similar chemical properties due to their identical number of valence electrons. This similarity allows for predictable reactions and bonding patterns. For example, Group 1 elements (alkali metals) are highly reactive, readily losing one electron to achieve a stable electron configuration. Similarly, Group 17 elements (halogens) are also reactive, readily gaining one electron to achieve a stable configuration. The noble gases in Group 18, with full valence shells, are exceptionally unreactive.
Blocks: Subshells and Electron Configuration
The periodic table is further subdivided into blocks based on the subshell that the outermost electrons occupy. The s-block, encompassing Groups 1 and 2, comprises alkali metals and alkaline earth metals. The p-block, including Groups 13-18, contains a diverse range of elements, including many nonmetals, metalloids, and some metals. The d-block, comprising Groups 3-12 (transition metals), exhibits characteristic properties like variable oxidation states and the formation of colored compounds. Finally, the f-block, located separately at the bottom of the table, comprises the lanthanides and actinides, elements with partially filled f subshells. The f-block elements show highly similar chemical properties within each series.
Key Features and Trends
The arrangement of the periodic table reveals several important periodic trends that govern the chemical and physical properties of elements. These trends are directly related to the electron configuration and effective nuclear charge.
Atomic Radius: Size Matters
Atomic radius, the distance from the nucleus to the outermost electron, generally decreases across a period (left to right) due to increasing nuclear charge. The added protons pull the electrons closer. Down a group (top to bottom), atomic radius increases as electrons are added to higher energy levels further from the nucleus.
Ionization Energy: Holding on to Electrons
Ionization energy is the energy required to remove an electron from an atom. It generally increases across a period due to the stronger attraction between the nucleus and the electrons. Down a group, ionization energy decreases due to the increased distance between the nucleus and the outermost electrons.
Electronegativity: Electron Affinity
Electronegativity reflects an atom's ability to attract electrons in a chemical bond. It generally increases across a period and decreases down a group, mirroring the trends in ionization energy. Highly electronegative elements readily attract electrons, leading to polar bonds.
Metallic Character: A Spectrum of Properties
Metallic character refers to the properties associated with metals, including conductivity, malleability, and ductility. Metallic character generally decreases across a period as the atoms become smaller and hold onto their electrons more tightly. It increases down a group as atomic size increases and the outermost electrons are held less tightly.
Special Regions of the Periodic Table
The periodic table is not just a grid; it highlights specific regions with unique characteristics:
Alkali Metals (Group 1): Reactive Champions
Highly reactive metals with one valence electron, readily forming +1 ions. They react vigorously with water, producing hydrogen gas.
Alkaline Earth Metals (Group 2): Relatively Reactive
Reactive metals with two valence electrons, forming +2 ions. They are less reactive than alkali metals but still exhibit considerable reactivity.
Transition Metals (Groups 3-12): A Colorful Cast
Metals with partially filled d orbitals, exhibiting variable oxidation states and forming colored compounds. They often act as catalysts.
Halogens (Group 17): Highly Reactive Nonmetals
Nonmetals with seven valence electrons, readily gaining one electron to form -1 ions. They are highly reactive, forming salts with metals.
Noble Gases (Group 18): Inert Giants
Nonmetals with full valence shells, making them exceptionally unreactive. Their stability stems from their complete octet of electrons.
Metalloids: Bridging the Gap
Elements located along the staircase separating metals and nonmetals. They exhibit properties of both metals and nonmetals, making them crucial in semiconductor technology.
Lanthanides and Actinides: The Inner Transition Metals
These elements, with partially filled f orbitals, are placed separately at the bottom to avoid excessively widening the table. They show remarkable similarities in chemical properties within each series.
Applications and Importance
The periodic table is far more than a chart; it's a dynamic tool that fuels innovation across various fields. Its insights allow scientists to:
- Predict chemical properties: Understanding trends allows scientists to anticipate how elements will behave in reactions.
- Design new materials: The table informs the development of materials with specific properties for technological applications.
- Understand biological systems: The essential elements for life are readily identifiable within the table.
- Develop new technologies: The unique properties of different elements fuel advancements in various industries.
- Educate and inspire: The periodic table serves as a foundational tool for chemistry education and scientific exploration.
Conclusion: A Continuing Story
The periodic table, born from careful observation and ingenious organization, continues to evolve as our understanding of matter deepens. New isotopes and elements are discovered, further enriching its complexity. Its elegant arrangement, however, remains a testament to the underlying order and predictability within the seemingly chaotic world of chemical elements. The periodic table stands as a symbol of scientific discovery, a powerful tool for understanding the universe, and a source of ongoing inspiration for scientific inquiry. The arrangement, constantly refined and expanded upon, will undoubtedly continue to shape our understanding of the fundamental building blocks of the cosmos for generations to come.
Latest Posts
Latest Posts
-
How To Calculate Freezing Point Of A Solution
Mar 28, 2025
-
What Is The Monomer That Makes Up Dna
Mar 28, 2025
-
Covalent Bonds Are Formed Between Two Non Metals
Mar 28, 2025
-
In General Pathogens Grow Very Slowly
Mar 28, 2025
-
Mixing An Acid And A Base
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about The Arrangement Of The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
