The Energy Required To Initiate An Exergonic Reaction Is Called
Muz Play
Mar 22, 2025 · 6 min read
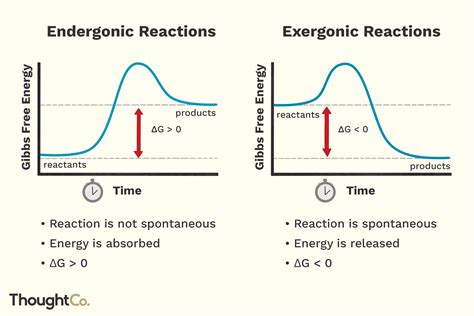
Table of Contents
The Energy Required to Initiate an Exergonic Reaction is Called Activation Energy
The concept of activation energy is fundamental to understanding chemical reactions, particularly those that release energy, known as exergonic reactions. While exergonic reactions have a negative Gibbs free energy change (ΔG), indicating a net release of energy, they still require an initial input of energy to get started. This initial energy "push" is what we call activation energy. Understanding activation energy is crucial in various fields, including chemistry, biology, and materials science. This article will delve deep into the concept of activation energy, exploring its significance, the factors influencing it, and its implications in different contexts.
What is Activation Energy?
Activation energy (Ea) is the minimum amount of energy required to initiate a chemical reaction. Think of it as the energy barrier that reactants must overcome to transform into products. Even though a reaction may be thermodynamically favorable (exergonic, meaning it releases energy overall), the reactants need a certain amount of energy to reach a transitional state, called the transition state or activated complex. This transition state is a high-energy, unstable intermediate form between reactants and products.
Imagine pushing a ball up a hill. The ball represents the reactants, the hill represents the activation energy barrier, and the valley on the other side represents the products. You need to exert energy to push the ball up the hill (reach the transition state), after which it rolls down the hill on its own, releasing energy as it goes (exergonic reaction). The energy you put in to push the ball uphill is analogous to the activation energy.
In simpler terms: Activation energy is the energy needed to break the existing bonds in the reactants and initiate the formation of new bonds in the products. Once this energy barrier is overcome, the reaction proceeds spontaneously, releasing the net energy difference between reactants and products.
Activation Energy and Exergonic Reactions: A Deeper Dive
Exergonic reactions, by definition, release energy. The energy released is often in the form of heat, light, or other forms of energy. However, the fact that a reaction is exergonic doesn't mean it will happen spontaneously at a noticeable rate. Many exergonic reactions have high activation energies, meaning they require a significant energy input to initiate the reaction, even though they will ultimately release energy.
The Transition State Theory
The transition state theory provides a framework for understanding how activation energy affects reaction rates. According to this theory, the reaction rate is proportional to the number of molecules that possess sufficient energy to overcome the activation energy barrier and reach the transition state. The higher the activation energy, the fewer molecules will have enough energy, resulting in a slower reaction rate.
The transition state itself is incredibly short-lived and unstable. It's a fleeting arrangement of atoms that represents the highest energy point along the reaction pathway. Studying the transition state provides insights into the reaction mechanism and allows scientists to design catalysts that lower the activation energy.
Factors Affecting Activation Energy
Several factors can influence the activation energy of a reaction:
1. Nature of Reactants:
The type of bonds in the reactants significantly impacts the activation energy. Stronger bonds require more energy to break, leading to a higher activation energy. For example, breaking a triple bond requires significantly more energy than breaking a single bond. The inherent reactivity of the reactants also plays a role. Highly reactive molecules generally have lower activation energies compared to less reactive ones.
2. Concentration of Reactants:
Increasing the concentration of reactants increases the frequency of collisions between them. More frequent collisions increase the likelihood of successful collisions (collisions with sufficient energy to overcome the activation energy barrier), leading to a faster reaction rate. However, the activation energy itself remains unchanged.
3. Temperature:
Temperature is a crucial factor affecting reaction rates. Increasing the temperature increases the kinetic energy of the reactant molecules. This leads to more frequent and more energetic collisions, increasing the proportion of molecules with sufficient energy to surpass the activation energy barrier. Consequently, the reaction rate increases exponentially with temperature. However, temperature does not change the activation energy itself.
4. Catalysts:
Catalysts are substances that increase the rate of a reaction without being consumed in the process. They achieve this by lowering the activation energy. Catalysts provide an alternative reaction pathway with a lower energy barrier, making it easier for reactants to reach the transition state. Enzymes, biological catalysts, are prime examples of this, enabling numerous biochemical reactions to occur at feasible rates under physiological conditions.
5. Surface Area:
For heterogeneous reactions (reactions involving reactants in different phases, e.g., a solid reacting with a gas), the surface area of the solid reactant plays a significant role. A larger surface area provides more sites for the reaction to occur, increasing the frequency of successful collisions and thus accelerating the reaction rate. Again, the activation energy itself is unaffected.
Activation Energy and Reaction Rate: The Arrhenius Equation
The relationship between activation energy, temperature, and reaction rate is quantitatively described by the Arrhenius equation:
k = A * e^(-Ea/RT)
Where:
- k is the rate constant (a measure of reaction rate)
- A is the pre-exponential factor (related to the frequency of collisions)
- Ea is the activation energy
- R is the gas constant
- T is the temperature in Kelvin
This equation shows the exponential dependence of the rate constant (and therefore the reaction rate) on the activation energy and temperature. A lower activation energy leads to a higher rate constant and a faster reaction rate. An increase in temperature also increases the rate constant.
Activation Energy in Different Contexts
The concept of activation energy is far-reaching, with applications across various scientific fields:
1. Chemistry:
Activation energy is crucial in understanding reaction mechanisms and kinetics. It helps predict reaction rates and design efficient reaction conditions. In industrial chemistry, optimizing reaction conditions to minimize activation energy is vital for maximizing product yield and efficiency.
2. Biology:
In biological systems, enzymes act as catalysts, lowering the activation energy of biochemical reactions. Without enzymes, many essential biological processes would occur too slowly to sustain life. Understanding enzyme kinetics and their activation energies is critical in studying metabolism, drug design, and disease mechanisms.
3. Materials Science:
Activation energy plays a key role in various materials processes, such as sintering (the consolidation of powder materials), crystal growth, and diffusion. Controlling activation energy through doping or other modifications allows for tailoring material properties.
4. Combustion:
Combustion reactions require a specific activation energy, usually provided by an ignition source (like a spark or flame). The activation energy determines the ease of ignition and the rate of combustion.
5. Environmental Science:
Understanding activation energies is crucial for studying environmental processes like atmospheric reactions and pollutant degradation. The activation energies of these reactions influence the lifetime of pollutants in the environment.
Conclusion
Activation energy is a critical concept that explains why even exergonic reactions require an initial energy input to proceed. It's not merely an abstract concept; it has profound implications across numerous disciplines. By understanding the factors that influence activation energy and employing strategies to manipulate it (e.g., using catalysts), we can control reaction rates and design efficient processes in chemistry, biology, materials science, and many other fields. The Arrhenius equation provides a mathematical framework for quantifying this relationship, providing powerful tools for prediction and optimization. Further research into activation energy and its associated factors continues to unveil new insights and applications, solidifying its importance in scientific understanding and technological advancement.
Latest Posts
Latest Posts
-
Effective Nuclear Charge Zeff Is Defined As
Mar 22, 2025
-
If Q Is Less Than K
Mar 22, 2025
-
Is Childbirth Positive Or Negative Feedback
Mar 22, 2025
-
How Was The Modern Periodic Table Arranged
Mar 22, 2025
-
Openings That Allow For Gas Exchange
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about The Energy Required To Initiate An Exergonic Reaction Is Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
