The Movement Of Water Through A Selectively Permeable Membrane
Muz Play
Mar 30, 2025 · 6 min read
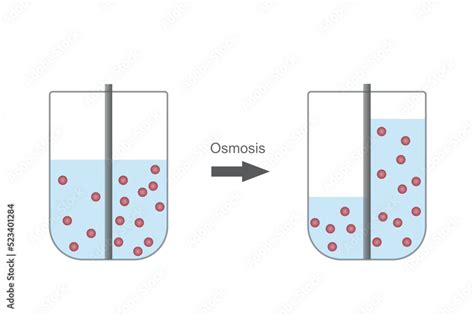
Table of Contents
The Movement of Water Through a Selectively Permeable Membrane: Osmosis Explained
The movement of water across a selectively permeable membrane is a fundamental process in biology, crucial for maintaining life at the cellular and organismal levels. This movement, known as osmosis, is driven by the difference in water potential between two solutions separated by the membrane. Understanding osmosis is key to comprehending a wide range of biological phenomena, from nutrient uptake in plants to the regulation of blood pressure in animals. This comprehensive guide will delve deep into the mechanics of osmosis, exploring its driving forces, influencing factors, and crucial biological roles.
What is a Selectively Permeable Membrane?
Before delving into the intricacies of osmosis, let's define the key player: the selectively permeable membrane. This is a biological membrane that allows certain substances to pass through while restricting others. The selective permeability arises from the membrane's structure, primarily composed of a phospholipid bilayer interspersed with proteins. This structure acts as a barrier, regulating the passage of molecules based on their size, charge, and polarity. Small, nonpolar molecules like oxygen and carbon dioxide can readily diffuse across the membrane, while larger, polar molecules like glucose and ions require specialized transport mechanisms. Water, while polar, is small enough to pass through the membrane albeit at a relatively slow rate, primarily through aquaporins.
The Role of Aquaporins
Aquaporins are integral membrane proteins that form channels specifically for water molecules. They significantly increase the permeability of the membrane to water, allowing for rapid water transport across the cell membrane. The presence and number of aquaporins in a membrane can greatly influence the rate of osmosis. The regulation of aquaporin expression and activity plays a crucial role in various physiological processes, including water balance and plant growth.
Understanding Osmosis: The Driving Force
Osmosis is the net movement of water across a selectively permeable membrane from a region of higher water potential to a region of lower water potential. This movement continues until equilibrium is reached, meaning the water potential on both sides of the membrane is equal. But what exactly is water potential?
Water potential is the measure of the free energy of water. It's influenced by several factors, primarily:
-
Solute potential (Ψs): This is the effect of dissolved solutes on the water potential. The presence of solutes lowers the water potential because they bind to water molecules, reducing the amount of free water available to move. A higher solute concentration results in a lower solute potential (more negative).
-
Pressure potential (Ψp): This refers to the physical pressure exerted on the water. Positive pressure potential (e.g., turgor pressure in plant cells) increases water potential, while negative pressure potential (e.g., tension in xylem vessels) decreases it.
The total water potential (Ψ) is the sum of solute potential and pressure potential: Ψ = Ψs + Ψp.
Osmosis in Action: Different Scenarios
Let's consider various scenarios to understand how osmosis plays out under different conditions:
1. Isotonic Solution: Equilibrium Achieved
When a cell is placed in an isotonic solution, the water potential inside the cell is equal to the water potential outside. There is no net movement of water across the membrane, and the cell maintains its shape. This is a state of equilibrium.
2. Hypotonic Solution: Water Influx and Potential Lysis
If a cell is placed in a hypotonic solution, the water potential outside the cell is higher than inside. Water moves into the cell by osmosis, causing it to swell. In animal cells, this can lead to lysis (cell bursting) if the influx of water is excessive. Plant cells, however, have a rigid cell wall that prevents lysis; instead, they become turgid, a state of firmness that contributes to plant support.
3. Hypertonic Solution: Water Efflux and Plasmolysis
In a hypertonic solution, the water potential outside the cell is lower than inside. Water moves out of the cell by osmosis, causing it to shrink. Animal cells undergo crenation (shrinking and shriveling), while plant cells experience plasmolysis, where the cell membrane pulls away from the cell wall. This process can severely impact cell function.
The Significance of Osmosis in Biological Systems
Osmosis plays a vital role in numerous biological processes across various organisms:
1. Water Uptake in Plants
Plants rely heavily on osmosis for water uptake from the soil. Water moves from the soil (high water potential) into the roots (lower water potential) and then travels up the xylem vessels to the leaves. This process is facilitated by the root hairs, which greatly increase the surface area for water absorption. The turgor pressure generated by osmosis maintains the rigidity of plant cells, keeping the plant upright and supporting its structure.
2. Nutrient Uptake in Plants
Osmosis isn't just about water; it also plays a critical role in the uptake of nutrients dissolved in the soil water. These nutrients are passively transported into the roots along with water, a process that's driven by the water potential gradient.
3. Maintaining Cell Shape and Volume
Osmosis helps maintain the appropriate cell shape and volume in both plant and animal cells. The constant regulation of water movement prevents excessive swelling or shrinkage, ensuring optimal cellular function.
4. Blood Pressure Regulation in Animals
In animals, osmosis is critical for regulating blood pressure and fluid balance. The kidneys play a vital role in controlling the concentration of solutes in the blood, which directly influences the water potential and thus, the blood volume. This, in turn, affects blood pressure.
5. Nutrient and Waste Exchange in Animals
Osmosis plays a crucial role in the exchange of nutrients and waste products between the blood and surrounding tissues. The movement of water across capillary walls helps maintain fluid balance and facilitates the transport of essential substances.
6. Water Balance in Single-celled Organisms
For single-celled organisms like protists, osmosis is essential for maintaining their internal water balance in different environments. They use various mechanisms, such as contractile vacuoles, to regulate water uptake and expulsion.
Factors Affecting Osmosis Rate
Several factors influence the rate of osmosis:
-
Concentration Gradient: A steeper concentration gradient (larger difference in water potential) leads to a faster rate of osmosis.
-
Temperature: Higher temperatures generally increase the rate of osmosis due to increased kinetic energy of water molecules.
-
Surface Area: A larger surface area for water movement (e.g., more root hairs in plants) results in faster osmosis.
-
Membrane Permeability: Membranes with higher water permeability (e.g., those with more aquaporins) facilitate faster water movement.
-
Thickness of Membrane: A thinner membrane allows for faster osmosis compared to a thicker membrane.
Osmosis and Reverse Osmosis: A Comparison
Reverse osmosis is a technology that leverages the principles of osmosis but in a reversed manner. While osmosis involves the natural movement of water from a region of high water potential to a region of low water potential, reverse osmosis uses external pressure to force water across a semipermeable membrane against its natural concentration gradient. This technology is widely used for water purification, desalination, and other applications.
Conclusion
Osmosis is a fundamental biological process with far-reaching consequences for living organisms. Its influence extends from the microscopic level of individual cells to the macroscopic level of entire ecosystems. Understanding the principles of osmosis, including its driving forces, influencing factors, and biological roles, is crucial for comprehending the intricate mechanisms that govern life. The constant interplay of water potential, solute potential, and pressure potential drives the movement of water across selectively permeable membranes, shaping the form, function, and survival of countless living organisms. Further research continues to uncover the intricate details of this vital process and its impact on various biological systems.
Latest Posts
Latest Posts
-
Active Sites On The Actin Become Available For Binding After
Apr 01, 2025
-
Is Sodium Methoxide A Strong Nucleophile
Apr 01, 2025
-
Which Statement About Immigration Federalism Is False
Apr 01, 2025
-
Which Will Increase The Rate Of A Chemical Reaction
Apr 01, 2025
-
How Are Electrons Arranged Around The Nucleus Of An Atom
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about The Movement Of Water Through A Selectively Permeable Membrane . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
