The Products Of A Combustion Reaction Do Not Include ____.
Muz Play
Mar 22, 2025 · 5 min read
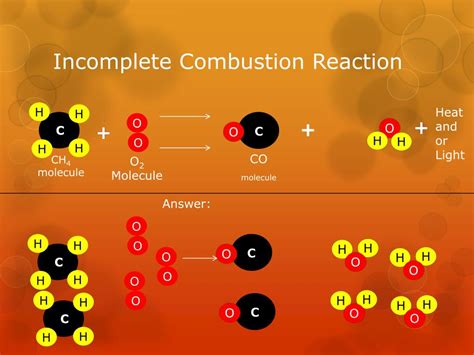
Table of Contents
The Products of a Combustion Reaction Do Not Include… Unburnt Reactants!
Combustion, a fundamental chemical process, is the rapid oxidation of a substance, typically involving a fuel and an oxidant, producing heat and light. While seemingly simple, understanding the nuances of combustion reactions and their products is crucial across numerous fields, from engine design to environmental science. This comprehensive exploration delves deep into the products of combustion, highlighting what's not included and the factors influencing the reaction's outcome.
Understanding the Basics of Combustion
Before diving into what's absent from combustion products, let's solidify our understanding of the process itself. Combustion reactions are fundamentally exothermic, meaning they release energy in the form of heat. The most common type involves the reaction of a fuel (like hydrocarbons in gasoline or wood) with an oxidant (usually oxygen in the air). This reaction produces various products, primarily determined by the fuel's composition and the availability of oxygen.
Essential Components of a Combustion Reaction:
-
Fuel: The substance undergoing oxidation, providing the energy source for the reaction. Examples include methane (CH₄), propane (C₃H₈), gasoline (a mixture of hydrocarbons), wood (cellulose and lignin), and even certain metals like magnesium.
-
Oxidant: The substance that accepts electrons during the oxidation process. Oxygen (O₂) is the most common oxidant, but others exist, such as chlorine (Cl₂) or fluorine (F₂).
-
Heat: Initiates the reaction and is also released as a product. The activation energy is needed to start the combustion process, often provided by a spark or flame.
The Primary Products of Complete Combustion
Complete combustion, occurring under ideal conditions with ample oxygen, results in predictable products:
-
Carbon Dioxide (CO₂): When a hydrocarbon fuel burns completely, all carbon atoms in the fuel combine with oxygen to form carbon dioxide. This is the most common and significant product of complete combustion of carbon-containing fuels.
-
Water (H₂O): If the fuel contains hydrogen (as most hydrocarbon fuels do), it reacts with oxygen to produce water vapor.
-
Heat (Energy): The primary driving force behind combustion, released as thermal energy.
Example: The complete combustion of methane (CH₄) can be represented as:
CH₄ + 2O₂ → CO₂ + 2H₂O + Heat
What the Products of Combustion Do Not Include (Under Ideal Conditions)
The key to understanding what's absent from a complete combustion reaction lies in the complete oxidation of the fuel. Under ideal conditions, there should be:
-
**No Unburnt Fuel: In complete combustion, all the fuel molecules react with oxygen. The absence of unburnt fuel indicates efficient combustion.
-
No Carbon Monoxide (CO): Carbon monoxide is a toxic byproduct of incomplete combustion. Its presence indicates insufficient oxygen to fully oxidize the carbon atoms to carbon dioxide.
-
No Soot (Elemental Carbon): Soot, composed of tiny particles of elemental carbon, is another sign of incomplete combustion. Insufficient oxygen prevents the complete oxidation of carbon atoms.
-
No Unreacted Oxygen: While oxygen is a reactant, in a stoichiometrically balanced reaction (meaning the exact amounts of fuel and oxygen are present), there shouldn't be any unreacted oxygen left after combustion. However, in reality, excess oxygen is often used to ensure complete combustion.
-
No Nitrogen Oxides (NOx): Although not directly related to the fuel-oxidant reaction itself, NOx formation is heavily influenced by combustion temperature and the presence of nitrogen in the air. Efficient combustion strategies can minimize NOx emissions.
Incomplete Combustion and its Products
The absence of the aforementioned byproducts hinges on the assumption of complete combustion. However, in reality, several factors can lead to incomplete combustion, yielding undesirable products:
-
Insufficient Oxygen: The most common cause of incomplete combustion. When oxygen is limited, carbon atoms may not fully oxidize to CO₂, resulting in CO and soot formation.
-
Poor Mixing: Inadequate mixing of fuel and air hinders efficient combustion, leading to localized areas with oxygen deficiency.
-
Low Temperature: Insufficient temperature can prevent the activation energy required for complete oxidation, resulting in incomplete combustion products.
Products of Incomplete Combustion:
-
Carbon Monoxide (CO): A highly toxic gas, odorless and colorless, resulting from incomplete oxidation of carbon.
-
Soot (Elemental Carbon): Fine particles of carbon, contributing to air pollution and respiratory problems.
-
Unburnt Hydrocarbons (UHC): Unreacted fuel molecules escaping the combustion process.
-
Other Partially Oxidized Compounds: Depending on the fuel, incomplete combustion might produce aldehydes, ketones, and other partially oxidized organic compounds.
Factors Affecting Combustion Products
Several factors influence the products of a combustion reaction, moving beyond the simple ideal scenario:
-
Fuel Type: The chemical composition of the fuel dictates the potential products. Different fuels produce different ratios of CO₂, H₂O, CO, soot, and other byproducts.
-
Oxygen Availability: As discussed, oxygen availability is paramount. Insufficient oxygen dramatically affects the reaction's outcome.
-
Temperature: The reaction temperature directly impacts the rate and completeness of combustion. Higher temperatures typically favor complete combustion.
-
Pressure: Pressure can influence the reaction rate and the equilibrium between different products.
-
Presence of Catalysts: Catalysts can affect the reaction pathway and influence the products formed.
-
Residence Time: The time the fuel and oxidant spend in the combustion zone influences the completeness of the reaction.
Environmental Implications of Incomplete Combustion
The products of incomplete combustion have significant environmental consequences:
-
Air Pollution: CO, soot, and UHC contribute to smog, acid rain, and respiratory illnesses.
-
Greenhouse Effect: CO₂ is a greenhouse gas contributing to climate change. While inevitable in complete combustion, minimizing CO₂ emissions is crucial.
-
Climate Change: Incomplete combustion releases additional greenhouse gases like methane and other volatile organic compounds that contribute to the greenhouse effect.
Conclusion: Understanding the Absence of Unburnt Reactants
The products of a combustion reaction, in the ideal scenario of complete combustion, do not include significant quantities of unburnt fuel, carbon monoxide, soot, or other partially oxidized compounds. The presence of these substances signifies incomplete combustion, highlighting the importance of understanding factors that influence the reaction's efficiency and the environmental impact of inefficient burning processes. Optimizing combustion processes to achieve complete combustion is critical for minimizing air pollution, reducing greenhouse gas emissions, and improving overall environmental sustainability. This requires careful consideration of fuel type, oxygen supply, temperature, pressure, and reaction time. Through advanced engineering and a deeper understanding of combustion chemistry, we can strive for cleaner and more efficient energy production.
Latest Posts
Latest Posts
-
Do Diastereomers Have The Same Physical Properties
Mar 24, 2025
-
Shear Force And Bending Moment Cantilever Beam
Mar 24, 2025
-
Do Nonmetals Have High Ionization Energy
Mar 24, 2025
-
During Meiosis But Not During Mitosis
Mar 24, 2025
-
How To Draw A Frequency Table In Excel
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about The Products Of A Combustion Reaction Do Not Include ____. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
