This Type Of Reaction Is Spontaneous And Releases Energy
Muz Play
Mar 20, 2025 · 6 min read
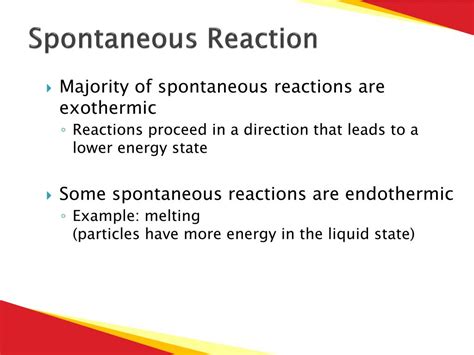
Table of Contents
Spontaneous Reactions: When Energy is Released
Spontaneous reactions are a cornerstone of chemistry and physics, underpinning countless natural processes and technological applications. This type of reaction, by definition, occurs without external intervention and is characterized by the release of energy into the surroundings. This released energy can manifest in various forms, including heat, light, and sound, and is often the driving force behind many impactful phenomena. Understanding the spontaneity of a reaction requires delving into thermodynamics, a branch of science focused on energy and its transformations.
Understanding Spontaneity: Gibbs Free Energy and its Implications
The spontaneity of a reaction is not solely dictated by whether it releases energy (exothermic) or absorbs energy (endothermic). While exothermic reactions often appear spontaneous, the true determinant is a thermodynamic quantity called Gibbs Free Energy (ΔG). Gibbs Free Energy combines enthalpy (ΔH, a measure of heat content) and entropy (ΔS, a measure of disorder or randomness) to predict the likelihood of a reaction proceeding spontaneously. The equation defining Gibbs Free Energy is:
ΔG = ΔH - TΔS
where:
- ΔG is the change in Gibbs Free Energy
- ΔH is the change in enthalpy
- T is the absolute temperature (in Kelvin)
- ΔS is the change in entropy
A negative ΔG value indicates a spontaneous reaction, meaning the reaction will proceed without external input of energy. Conversely, a positive ΔG value signifies a non-spontaneous reaction, requiring energy input to occur. A ΔG of zero indicates a reaction at equilibrium, where the forward and reverse reactions occur at equal rates.
The Role of Enthalpy (ΔH)
Enthalpy (ΔH) represents the heat absorbed or released during a reaction at constant pressure. A negative ΔH (exothermic reaction) indicates that heat is released to the surroundings, while a positive ΔH (endothermic reaction) means heat is absorbed from the surroundings. Exothermic reactions often feel warm to the touch, while endothermic reactions often feel cool.
The Role of Entropy (ΔS)
Entropy (ΔS) describes the degree of disorder or randomness in a system. Reactions that lead to an increase in disorder (positive ΔS) tend to be more spontaneous. For example, the melting of ice (solid to liquid) is spontaneous because the liquid state is more disordered than the solid state. Conversely, a decrease in disorder (negative ΔS) opposes spontaneity.
Temperature's Influence
The absolute temperature (T) plays a crucial role in determining spontaneity. The TΔS term in the Gibbs Free Energy equation indicates that the contribution of entropy to spontaneity increases with increasing temperature. At high temperatures, the entropic contribution can overcome an unfavorable enthalpy change, making an endothermic reaction spontaneous.
Examples of Spontaneous Reactions and Energy Release
Many everyday processes involve spontaneous reactions that release energy. Here are some notable examples:
Combustion
Combustion, the rapid reaction of a substance with oxygen, is a highly spontaneous and exothermic process. The burning of wood, natural gas, or gasoline are all examples of combustion reactions that release significant amounts of heat and light. The negative ΔH and positive ΔS values combine to result in a large negative ΔG.
Keywords: combustion, exothermic reaction, heat release, energy release, spontaneous process
Respiration
Cellular respiration, the process by which living organisms convert glucose into energy, is another example of a spontaneous and exothermic reaction. This complex series of reactions releases energy in the form of ATP (adenosine triphosphate), the primary energy currency of cells. While intricate, the overall reaction shows a substantial decrease in Gibbs Free Energy.
Keywords: respiration, cellular respiration, ATP, energy production, metabolism, exothermic
Neutralization Reactions
The reaction between an acid and a base, known as a neutralization reaction, is often spontaneous and exothermic. The formation of water and a salt releases heat, contributing to the negative ΔH. The increase in disorder associated with the formation of ions in solution also contributes to a positive ΔS, resulting in a negative ΔG.
Keywords: neutralization reaction, acid-base reaction, exothermic, heat of neutralization, spontaneous reaction, salt formation
Radioactive Decay
Radioactive decay is a spontaneous process where unstable atomic nuclei release energy in the form of particles or electromagnetic radiation. This process is driven by the tendency of the nucleus to achieve a more stable configuration, and the energy released appears as kinetic energy of the emitted particles and radiation. The decrease in mass is accompanied by a large negative ΔH, despite the entropy change being relatively small.
Keywords: radioactive decay, nuclear reaction, energy release, alpha decay, beta decay, gamma decay, spontaneous process
Non-Spontaneous Reactions and the Need for Energy Input
While many reactions are spontaneous, many others are non-spontaneous (ΔG > 0), requiring an external energy source to proceed. These reactions often involve an increase in order (negative ΔS) or a significant absorption of heat (positive ΔH). Examples include:
Photosynthesis
Photosynthesis, the process by which plants convert light energy into chemical energy, is a prime example of a non-spontaneous reaction. It requires energy input from sunlight to drive the endothermic synthesis of glucose from carbon dioxide and water. The increase in order and the positive enthalpy change are offset by the energy input from light.
Keywords: photosynthesis, endothermic reaction, energy input, light energy, chemical energy, glucose synthesis
Electrolysis
Electrolysis is the process of using electrical energy to drive a non-spontaneous chemical reaction. For example, the electrolysis of water to produce hydrogen and oxygen requires a direct current power source to overcome the positive ΔG of the reaction.
Keywords: electrolysis, non-spontaneous reaction, electrical energy, chemical energy, water electrolysis, hydrogen production, oxygen production
Protein Synthesis
Protein synthesis, the process of creating proteins from amino acids, requires energy input in the form of ATP. The assembly of amino acids into a specific sequence is a highly ordered process, resulting in a significant decrease in entropy (negative ΔS). The energy released by the hydrolysis of ATP fuels this unfavorable process.
Keywords: protein synthesis, non-spontaneous reaction, ATP hydrolysis, amino acid polymerization, energy consumption
Factors Affecting the Rate of Spontaneous Reactions
While spontaneity predicts whether a reaction will occur, it does not determine how fast it will occur. The rate of a spontaneous reaction depends on various factors, including:
- Activation Energy: Even spontaneous reactions require an initial input of energy, called the activation energy, to initiate the reaction. A lower activation energy results in a faster reaction rate.
- Concentration of Reactants: Higher concentrations generally lead to faster reaction rates due to increased collision frequency.
- Temperature: Increasing temperature usually increases the reaction rate by increasing the kinetic energy of the reactants and the frequency of successful collisions.
- Presence of a Catalyst: Catalysts lower the activation energy of a reaction, thereby increasing the reaction rate without being consumed in the process.
Applications of Spontaneous Reactions
Spontaneous reactions are utilized extensively in various applications:
- Energy Production: Combustion of fuels is a cornerstone of electricity generation.
- Industrial Processes: Numerous industrial processes rely on spontaneous reactions for the synthesis of various chemicals and materials.
- Batteries: Electrochemical cells utilize spontaneous redox reactions to generate electricity.
- Medical Applications: Many biological processes and medical treatments rely on spontaneous reactions.
Conclusion
Spontaneous reactions, characterized by their ability to proceed without external intervention and their release of energy, are fundamental to numerous natural and technological processes. While the release of energy (exothermic nature) is often associated with spontaneity, the Gibbs Free Energy (ΔG) provides the definitive criterion, considering both enthalpy and entropy changes. Understanding spontaneity requires careful consideration of thermodynamic principles, offering valuable insights into the driving forces behind countless chemical and physical transformations. The factors affecting the rate of spontaneous reactions, such as activation energy, concentration, temperature and catalysis, further enhance our ability to harness and manipulate these transformative processes for various practical applications. The study of spontaneous reactions remains a dynamic field, continuously revealing new insights and driving innovation across diverse scientific disciplines.
Latest Posts
Latest Posts
-
Is Carbon Dioxide A Pure Substance
Mar 21, 2025
-
Map Of North Africa Southwest Asia
Mar 21, 2025
-
What Is The Function Of Stem
Mar 21, 2025
-
What Is The Property Of A Base
Mar 21, 2025
-
How To Find The Excess Reactant
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about This Type Of Reaction Is Spontaneous And Releases Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
