Trend Of Electronegativity In The Periodic Table
Muz Play
Mar 16, 2025 · 5 min read
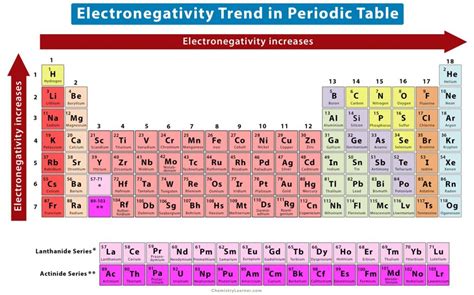
Table of Contents
The Trend of Electronegativity in the Periodic Table: A Comprehensive Guide
Electronegativity, a fundamental concept in chemistry, dictates how strongly an atom attracts electrons within a chemical bond. Understanding its trends across the periodic table is crucial for predicting molecular polarity, bond types, and overall chemical reactivity. This comprehensive guide delves into the intricacies of electronegativity, exploring its definition, measurement, periodic trends, and exceptions, providing a robust foundation for anyone seeking to master this essential chemical concept.
Defining Electronegativity: A Measure of Electron Attraction
Electronegativity quantifies an atom's ability to attract shared electrons in a covalent bond. It's not a directly measurable property like mass or charge, but rather a relative value, typically expressed on a scale. The most widely used scale is the Pauling scale, where fluorine, the most electronegative element, is assigned a value of 4.0. Other elements are then assigned values relative to fluorine. A higher electronegativity value indicates a stronger pull on electrons. This difference in electronegativity between atoms within a bond is what leads to the formation of polar covalent bonds, where the electron density is unevenly distributed. If the difference is large enough, it can even result in the formation of an ionic bond, where electrons are essentially transferred from one atom to another.
Understanding the Scales: Pauling, Mulliken, and Allred-Rochow
While the Pauling scale is the most common, other electronegativity scales exist, each with its own approach to quantification. The Mulliken scale, for instance, defines electronegativity as the average of an atom's ionization energy (the energy required to remove an electron) and electron affinity (the energy released when an electron is added). The Allred-Rochow scale considers the effective nuclear charge experienced by valence electrons and the atomic radius. While different scales produce slightly varying numerical values, they all consistently reflect the same general trends across the periodic table.
Periodic Trends in Electronegativity: Across and Down
The periodic trends of electronegativity are largely driven by two key factors: effective nuclear charge and atomic radius.
Trend 1: Across a Period (Left to Right)
As we move from left to right across a period in the periodic table, electronegativity generally increases. This is because the number of protons in the nucleus increases, leading to a stronger positive charge that attracts electrons more effectively. While additional electrons are added to the same shell, the increasing nuclear charge outweighs the effect of increased electron-electron repulsion, resulting in a higher effective nuclear charge felt by the valence electrons. Consequently, atoms pull more strongly on shared electrons, leading to increased electronegativity.
Example: Across the second period, from lithium (Li) to fluorine (F), electronegativity steadily increases. Lithium has a relatively weak hold on its valence electron, while fluorine, with its high nuclear charge and small atomic radius, exerts a very strong pull.
Trend 2: Down a Group (Top to Bottom)
Moving down a group in the periodic table, electronegativity generally decreases. This is primarily due to the increasing atomic radius. As we go down a group, new electron shells are added, placing the valence electrons further from the nucleus. The increased distance diminishes the attractive force of the nucleus on these outer electrons, resulting in decreased electronegativity. The shielding effect of inner electrons also plays a role, reducing the effective nuclear charge felt by the valence electrons.
Example: In Group 17 (halogens), fluorine (F) is the most electronegative, followed by chlorine (Cl), bromine (Br), iodine (I), and astatine (At). The increasing atomic radius down the group leads to a decrease in electronegativity.
Exceptions to the General Trends: The Nuances of Electronegativity
While the general trends are quite reliable, some exceptions exist. These deviations often arise due to subtle variations in electron configurations, shielding effects, and other atomic properties.
The Case of Some Transition Metals
Transition metals exhibit more irregular electronegativity trends compared to main group elements. Their partially filled d orbitals can complicate electron interactions and shielding effects, making precise prediction more challenging.
The Role of Electron Configuration:
Atoms with filled or half-filled subshells sometimes show slightly higher electronegativity than expected based solely on atomic radius and nuclear charge. This stability associated with complete or half-complete subshells can enhance the atom's ability to attract electrons.
Predicting Bond Polarity Using Electronegativity
The difference in electronegativity between two atoms in a bond determines the bond's polarity.
- Nonpolar Covalent Bond: When the electronegativity difference is close to zero (generally less than 0.4), the electrons are shared relatively equally, resulting in a nonpolar covalent bond.
- Polar Covalent Bond: When the electronegativity difference is moderate (between 0.4 and 1.7), the electrons are shared unequally, creating a polar covalent bond with a partial positive (δ+) end and a partial negative (δ-) end.
- Ionic Bond: When the electronegativity difference is large (greater than 1.7), electrons are essentially transferred from the less electronegative atom to the more electronegative atom, forming an ionic bond.
Applications of Electronegativity: Beyond Basic Chemistry
Understanding electronegativity trends has far-reaching implications in various fields of chemistry and related disciplines:
-
Predicting Molecular Geometry: Electronegativity influences molecular geometry by affecting bond angles and molecular shapes. The uneven distribution of electron density in polar bonds can lead to bent or asymmetrical structures.
-
Understanding Chemical Reactivity: Electronegativity is a key factor influencing chemical reactions. Highly electronegative atoms tend to be strong oxidizing agents, readily accepting electrons from other atoms.
-
Designing New Materials: Researchers leverage electronegativity principles when designing new materials with specific properties. The selection of elements based on their electronegativity allows for tailoring of material characteristics like conductivity, reactivity, and strength.
-
Drug Design and Development: In medicinal chemistry, electronegativity plays a critical role in understanding drug-receptor interactions. The polarity of drug molecules, determined by the electronegativity of their constituent atoms, influences their binding affinity and efficacy.
Conclusion: Mastering Electronegativity for Chemical Understanding
Electronegativity is a cornerstone concept in chemistry. By grasping its definition, measurement, periodic trends, and exceptions, one gains a deeper understanding of chemical bonding, molecular properties, and reactivity. Its importance extends far beyond introductory chemistry, proving essential in diverse fields like materials science, biochemistry, and pharmacology. Understanding the nuances of electronegativity empowers scientists and students to predict chemical behavior and design new materials and molecules with tailored properties. The trends, though generally predictable, warrant careful consideration of the exceptions to ensure accurate predictions. Therefore, a comprehensive grasp of electronegativity is crucial for anyone seeking to excel in the study of chemistry.
Latest Posts
Latest Posts
-
Factors That Influence The Elasticity Of Supply
Mar 17, 2025
-
Describe How The Atoms In A Compound Are Held Together
Mar 17, 2025
-
How Is Absorbance Linked To Rate Of Reaction
Mar 17, 2025
-
What Happens To Electrons In An Ionic Bond
Mar 17, 2025
-
Are Antiparalell Beta Sheets Mrore Stable
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Trend Of Electronegativity In The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
