Volume Of A Bcc Unit Cell
Muz Play
Mar 24, 2025 · 5 min read
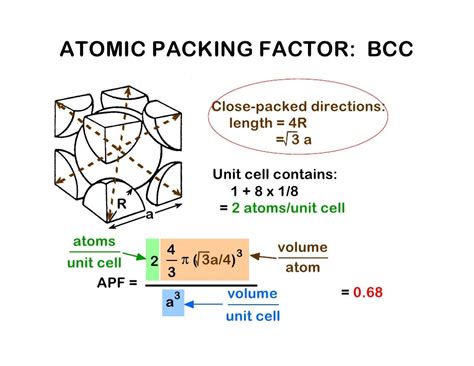
Table of Contents
Delving Deep into the Volume of a BCC Unit Cell: A Comprehensive Guide
The body-centered cubic (BCC) unit cell is a fundamental structure in crystallography, representing the arrangement of atoms in numerous metals and alloys. Understanding its volume is crucial for various applications, from materials science to nanotechnology. This comprehensive guide will explore the intricacies of calculating the BCC unit cell volume, delving into its theoretical underpinnings and practical implications. We'll cover everything from basic definitions to advanced considerations, ensuring a thorough understanding of this important concept.
Understanding the BCC Unit Cell Structure
Before calculating the volume, it's essential to visualize the BCC structure. Imagine a cube. In a BCC arrangement, one atom resides at each corner of this cube (eight corner atoms), and a single atom is situated at the very center of the cube (one central atom). This central atom is crucial in distinguishing a BCC structure from a simple cubic (SC) structure.
Key Characteristics of BCC:
- Coordination Number: Each atom in a BCC structure is surrounded by eight nearest neighbors. This is its coordination number.
- Number of Atoms per Unit Cell: While there are eight corner atoms, each is shared by eight adjacent unit cells. Therefore, each corner atom contributes 1/8 of an atom to the unit cell. Adding this to the single central atom, the total number of atoms per BCC unit cell is 2 (8 x 1/8 + 1 = 2).
- Atomic Packing Factor (APF): The APF represents the fraction of volume within the unit cell that is occupied by atoms. In BCC, the APF is approximately 0.68, indicating that a significant portion of the unit cell is empty space. This empty space influences many material properties.
Calculating the Volume of a BCC Unit Cell: A Step-by-Step Approach
The volume of a BCC unit cell is directly related to the atomic radius (r) of the constituent atoms. The calculation involves geometrical considerations within the cube.
1. Relating Atomic Radius to Unit Cell Edge Length:
The key to calculating the volume lies in understanding the relationship between the atomic radius (r) and the unit cell edge length (a). Consider a diagonal plane passing through the central atom and two opposite corner atoms. This diagonal forms a right-angled triangle within the unit cell. Applying the Pythagorean theorem in three dimensions, we get:
(4r)² = a² + a² + a² = 3a²
Solving for 'a', the unit cell edge length, yields:
a = 4r / √3
2. Calculating the Unit Cell Volume:
The volume (V) of a cube is simply the cube of its edge length:
V = a³ = (4r / √3)³ = 64r³ / 3√3
This formula provides the volume of the BCC unit cell as a function of the atomic radius. Therefore, knowing the atomic radius of the atoms forming the BCC structure allows for a straightforward calculation of the unit cell volume.
Practical Applications and Implications
The volume of the BCC unit cell has significant implications in various fields:
- Materials Science: Understanding the unit cell volume is crucial for determining material density. Density is directly proportional to the mass of atoms in the unit cell and inversely proportional to its volume. This information is essential for predicting material behavior under different conditions.
- Nanotechnology: In nanotechnology, controlling the size and structure of materials at the atomic level is vital. Precise calculations of unit cell volume are critical for designing and synthesizing nanomaterials with specific properties. The volume directly influences the number of atoms contained within a nanoparticle, consequently affecting its properties.
- Crystallography: The volume plays a critical role in diffraction studies. X-ray diffraction techniques rely on the interaction of X-rays with the crystal lattice. The unit cell volume directly influences the diffraction pattern, allowing determination of crystal structure.
- Metallurgy: Many metals and alloys exhibit BCC structures. Knowledge of the unit cell volume is crucial for understanding the mechanical properties of these materials, such as strength, ductility, and hardness. The relationship between volume and interstitial sites (spaces between atoms) influences the ability of the material to accommodate impurities or other atoms.
Advanced Considerations and Beyond the Basics
While the basic calculation provides a good approximation, several factors can influence the accuracy of the volume calculation:
- Thermal Expansion: Temperature fluctuations can cause changes in the unit cell dimensions, thus altering the volume. For accurate calculations at elevated temperatures, thermal expansion coefficients need to be considered.
- Pressure Effects: High pressure can compress the unit cell, reducing its volume. For precise calculations under high-pressure conditions, the compressibility of the material must be accounted for.
- Imperfections: Real crystals are not perfect; they contain defects like vacancies and dislocations. These imperfections can slightly alter the unit cell dimensions and volume. Advanced techniques like high-resolution microscopy are needed to accurately account for these defects.
- Alloying Effects: In alloys, the presence of different atomic species can lead to deviations from the ideal BCC structure and changes in unit cell volume. The size and distribution of different atoms within the lattice affect the overall volume.
Relating Volume to Other Properties: Density Calculation
One of the most practical applications of the BCC unit cell volume is in calculating the material's density (ρ). The density is given by:
ρ = (n x M) / (V x N<sub>A</sub>)
Where:
- n = number of atoms per unit cell (2 for BCC)
- M = atomic mass of the element
- V = volume of the unit cell (calculated as described above)
- N<sub>A</sub> = Avogadro's number (6.022 x 10²³ atoms/mol)
This formula allows us to connect the microscopic properties (atomic radius, unit cell volume) to the macroscopic property (density) of the material.
Conclusion: A Powerful Tool for Understanding Material Properties
The volume of a BCC unit cell is a fundamental concept with widespread applications across numerous scientific and engineering disciplines. Accurate calculation of this volume, taking into account the relevant factors discussed above, is essential for predicting and understanding material properties. From the simple geometric calculation to its advanced applications in materials science and nanotechnology, the BCC unit cell volume remains a powerful tool for characterizing and manipulating materials at the atomic level. Further research and refinement of calculation methods will undoubtedly enhance our understanding and ability to engineer materials with specific properties based on precise control over their unit cell volume. This comprehensive guide has aimed to equip readers with a thorough understanding of this critical parameter, enabling them to use this knowledge effectively in their respective fields.
Latest Posts
Latest Posts
-
Periodic Table With Metals Nonmetals And Metalloids
Mar 26, 2025
-
How To Find A Collision Force
Mar 26, 2025
-
Is Diethyl Ether Soluble In Water
Mar 26, 2025
-
Are D Sugars More Abundant In Nature Than L Sugars
Mar 26, 2025
-
Energy Is Released When Bonds Are Broken
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Volume Of A Bcc Unit Cell . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
