What Are 2 Components Of A Solution
Muz Play
Mar 26, 2025 · 6 min read
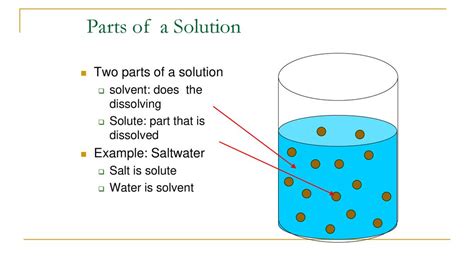
Table of Contents
What are the 2 Components of a Solution? A Deep Dive into Solutes and Solvents
Understanding the fundamental components of a solution is crucial in various scientific disciplines, from chemistry and biology to environmental science and materials engineering. While the concept may seem simple at first glance, a deeper exploration reveals a fascinating world of interactions and properties that shape our everyday lives. This article will delve into the two essential components of a solution: solutes and solvents, exploring their characteristics, interactions, and the factors that influence solubility.
The Two Pillars of a Solution: Solute and Solvent
At its core, a solution is a homogeneous mixture of two or more substances. This means the components are evenly distributed throughout the mixture, resulting in a uniform composition. The two fundamental components are:
- Solute: This is the substance that is being dissolved in a solution. It's typically present in a smaller amount compared to the solvent. Think of it as the substance that "disappears" into the solvent.
- Solvent: This is the substance that dissolves the solute. It's usually the component present in the larger amount. The solvent acts as the medium in which the solute particles are dispersed.
Let's explore each component in greater detail:
Understanding Solutes: A Closer Look at the Dissolved Substance
Solutes can exist in various forms, including solids, liquids, and gases. Their characteristics significantly influence their solubility in different solvents. Key factors determining solute behavior include:
1. Chemical Nature of the Solute:
The chemical structure and properties of the solute are paramount in determining its solubility. Polar solutes, which possess a significant difference in electronegativity between their atoms, tend to dissolve readily in polar solvents like water. This is due to the strong electrostatic interactions between the solute and solvent molecules. Examples of polar solutes include sugar (sucrose) and salt (sodium chloride).
Conversely, nonpolar solutes, with minimal differences in electronegativity, are more soluble in nonpolar solvents. These interactions are primarily driven by weaker van der Waals forces. Examples include oils and fats dissolving in organic solvents like hexane. This principle is encapsulated in the adage, "like dissolves like."
2. Particle Size of the Solute:
The size of the solute particles directly impacts the rate of dissolution. Smaller particles have a larger surface area to volume ratio, facilitating faster interaction with the solvent molecules. This is why grinding a solid solute into a fine powder accelerates its dissolution.
3. Temperature and Pressure Influence:
Temperature often plays a significant role in the solubility of solutes. For most solid solutes, solubility increases with increasing temperature. However, the relationship is not always linear and can vary depending on the specific solute and solvent. For gaseous solutes, solubility generally decreases with increasing temperature.
Pressure primarily affects the solubility of gaseous solutes. According to Henry's Law, the solubility of a gas in a liquid is directly proportional to the partial pressure of the gas above the liquid. This explains why carbonated beverages fizz more when opened, releasing pressure and reducing the solubility of carbon dioxide.
Deep Dive into Solvents: The Dissolving Medium
The solvent is the cornerstone of a solution, determining the ability to dissolve various solutes. Their properties significantly influence the solution's characteristics:
1. Polarity of the Solvent:
As mentioned earlier, the polarity of the solvent dictates the type of solute it can dissolve. Polar solvents like water effectively dissolve polar solutes due to strong dipole-dipole interactions and hydrogen bonding. Nonpolar solvents such as hexane dissolve nonpolar solutes through weaker London dispersion forces.
2. Solvent Strength:
Solvent strength, or solvating power, describes a solvent's ability to dissolve various solutes. This is determined by several factors including polarity, hydrogen bonding capacity, and dielectric constant. Strong solvents effectively solvate a wide range of solutes, while weaker solvents only dissolve a limited number of substances.
3. The Role of Hydrogen Bonding:
Hydrogen bonding, a special type of dipole-dipole interaction, plays a crucial role in the solvation of many polar molecules. Water, a highly effective solvent, owes its strong solvating power to its ability to form extensive hydrogen bonds with other polar molecules. This allows it to dissolve a vast array of substances, leading to its classification as a universal solvent.
Factors Affecting Solubility: A Complex Interplay
Solubility, the maximum amount of solute that can dissolve in a given amount of solvent at a specific temperature and pressure, is a complex phenomenon influenced by several interrelated factors:
1. Temperature:
As discussed earlier, temperature impacts solubility differently for solids and gases. For solid solutes, solubility generally increases with temperature, but exceptions exist. For gases, solubility typically decreases with increasing temperature.
2. Pressure:
Pressure mainly affects the solubility of gases. Higher pressure increases the solubility of gases in liquids, as described by Henry's Law. Pressure has a negligible effect on the solubility of solids and liquids.
3. Nature of Solute and Solvent:
The "like dissolves like" principle dictates that polar solutes dissolve in polar solvents, and nonpolar solutes dissolve in nonpolar solvents. This is because of the strong attractive forces between similar molecules.
4. Presence of Other Solutes:
The presence of other dissolved substances in the solvent can affect the solubility of a particular solute. Common-ion effect, for example, reduces the solubility of a sparingly soluble salt when a common ion is already present in the solution.
Applications of Understanding Solutions: A Wide Spectrum
The concept of solutions and the understanding of their components are vital in various fields:
- Medicine: Drug delivery relies heavily on understanding solubility. Drugs must dissolve in bodily fluids to be absorbed and exert their therapeutic effects.
- Environmental Science: Understanding the solubility of pollutants is essential for assessing their environmental impact and developing remediation strategies.
- Chemical Engineering: Many industrial processes involve solutions, requiring precise control over solute and solvent interactions for efficient production.
- Food Science: The solubility of various components determines the texture, flavor, and stability of food products.
- Materials Science: Solutions are used extensively in creating novel materials with specific properties, such as alloys and polymers.
Conclusion: A Foundation for Scientific Understanding
The seemingly simple concept of a solution, encompassing the two components—solute and solvent—lays the groundwork for understanding a wide range of scientific phenomena. The interplay between these components, influenced by various factors, determines the properties of solutions and their applications across numerous disciplines. A deep understanding of these fundamental principles is essential for advancements in science, technology, and beyond. From the mundane to the highly sophisticated, solutions and their constituent parts are fundamental building blocks of our world. Continued research and exploration in this area will undoubtedly lead to further innovations and discoveries, shaping our future in unexpected ways.
Latest Posts
Latest Posts
-
Curriculum Models In Early Childhood Education
Mar 26, 2025
-
Using Hesss Law To Calculate Net Reaction Enthalpy
Mar 26, 2025
-
Solids Have A Definite Shape Because
Mar 26, 2025
-
What Is A Subscript In A Chemical Equation
Mar 26, 2025
-
How To Do Post Closing Trial Balance
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Are 2 Components Of A Solution . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
