What Does Enzyme Binding Affinity Mean
Muz Play
Mar 29, 2025 · 6 min read
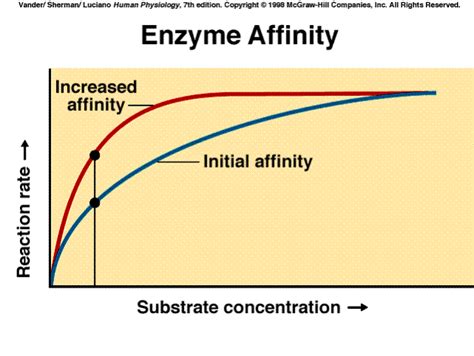
Table of Contents
What Does Enzyme Binding Affinity Mean? A Deep Dive into Enzyme-Substrate Interactions
Enzyme binding affinity is a crucial concept in biochemistry and enzymology, influencing the rate of enzymatic reactions and overall biological processes. Understanding this concept is vital for researchers, students, and anyone interested in the intricacies of biological systems. This article will provide a comprehensive overview of enzyme binding affinity, delving into its definition, measurement, factors influencing it, and its broader implications.
Defining Enzyme Binding Affinity
Enzyme binding affinity refers to the strength of the interaction between an enzyme and its substrate. It essentially describes how readily an enzyme binds to its target molecule (substrate) to form an enzyme-substrate complex. This interaction is a crucial first step in catalysis, the process by which enzymes accelerate biochemical reactions. A high binding affinity indicates a strong interaction, meaning the enzyme and substrate bind tightly together. Conversely, a low binding affinity suggests a weaker interaction, with the enzyme and substrate less likely to form a stable complex.
This binding isn't a random collision; it's a specific, highly selective process driven by various intermolecular forces, including:
- Hydrogen bonds: Electrostatic attraction between a hydrogen atom and a highly electronegative atom (like oxygen or nitrogen).
- Ionic interactions: Electrostatic attractions between oppositely charged groups.
- Hydrophobic interactions: The tendency of nonpolar molecules to cluster together in an aqueous environment.
- Van der Waals forces: Weak, short-range attractive forces between molecules.
The strength of these interactions collectively determines the overall binding affinity.
The Importance of Specificity
It's crucial to emphasize the specificity of enzyme-substrate interactions. Enzymes are highly selective, typically binding to only one or a very limited number of substrates. This specificity is primarily dictated by the enzyme's active site, a three-dimensional pocket or cleft on the enzyme's surface where the substrate binds. The active site's shape and chemical properties are perfectly complementary to the substrate, ensuring a precise and efficient interaction. This lock-and-key model, though simplified, highlights the importance of precise molecular recognition in enzyme function. The induced-fit model adds a layer of complexity, suggesting that the enzyme's active site can undergo conformational changes upon substrate binding, further optimizing the interaction.
Measuring Enzyme Binding Affinity
The strength of enzyme-substrate binding is quantitatively described by the dissociation constant (Kd). Kd represents the concentration of substrate at which half of the enzyme molecules are bound to substrate. A lower Kd value indicates a higher binding affinity, meaning the enzyme and substrate interact strongly, and a higher Kd value signifies a weaker interaction.
Several techniques are used to measure Kd:
-
Surface Plasmon Resonance (SPR): This technique monitors changes in the refractive index near a sensor surface as molecules bind and dissociate. It provides real-time information about binding kinetics and affinity.
-
Isothermal Titration Calorimetry (ITC): ITC measures the heat released or absorbed during binding interactions. It directly provides thermodynamic parameters such as Kd, enthalpy (ΔH), and entropy (ΔS), giving a comprehensive understanding of the binding process.
-
Fluorescence Spectroscopy: This method utilizes fluorescent probes to detect changes in fluorescence intensity upon substrate binding. It can provide information about binding affinity and also conformational changes in the enzyme.
-
Equilibrium Dialysis: This technique separates enzyme and substrate using a semi-permeable membrane, allowing the determination of the free and bound concentrations at equilibrium. This can be used to calculate the Kd value.
Factors Influencing Enzyme Binding Affinity
Numerous factors influence enzyme binding affinity. These include:
-
Substrate structure: The size, shape, and chemical properties of the substrate directly affect its ability to interact with the enzyme's active site. Minor changes in substrate structure can significantly alter binding affinity.
-
Enzyme structure: The conformation of the enzyme's active site, including the arrangement of amino acid residues, significantly influences substrate binding. Mutations or post-translational modifications can alter the active site structure and thus the binding affinity.
-
pH: The pH of the environment can influence the ionization state of amino acid residues in the active site, affecting electrostatic interactions and thus binding affinity. Each enzyme has an optimal pH range for maximal activity.
-
Temperature: Temperature affects the kinetic energy of molecules, influencing the rate of binding and dissociation. However, extreme temperatures can denature the enzyme, rendering it inactive.
-
Presence of inhibitors or activators: Inhibitors can compete with the substrate for binding to the active site, reducing the apparent binding affinity. Activators can enhance binding by inducing conformational changes in the enzyme.
-
Allosteric effects: Binding of a molecule to a site other than the active site (allosteric site) can induce conformational changes that affect substrate binding at the active site. This can either increase or decrease binding affinity.
-
Post-translational modifications: Modifications like phosphorylation or glycosylation can alter the enzyme's structure and consequently its binding affinity.
Implications of Enzyme Binding Affinity
Enzyme binding affinity has profound implications across various biological processes:
-
Metabolic regulation: The binding affinity of enzymes to their substrates plays a vital role in regulating metabolic pathways. Changes in binding affinity can alter the rate of enzymatic reactions, fine-tuning metabolic fluxes.
-
Drug design: Understanding enzyme binding affinity is crucial in drug development. Drugs often act as inhibitors, binding to the active site of enzymes to block their activity. High affinity inhibitors are more potent and effective.
-
Disease mechanisms: Many diseases are associated with alterations in enzyme binding affinity. Mutations affecting the active site can reduce binding affinity, leading to enzyme dysfunction and disease.
-
Biosensors: Enzyme binding affinity is exploited in the development of biosensors, devices that detect specific molecules by measuring changes in enzyme activity upon substrate binding.
-
Industrial biotechnology: Enzymes with high binding affinities are used in various industrial applications, such as biocatalysis and bioremediation.
The Dynamic Nature of Enzyme-Substrate Interactions
It's crucial to remember that enzyme-substrate interactions are dynamic. The enzyme and substrate are constantly binding and dissociating. The rate of these processes—association (k<sub>on</sub>) and dissociation (k<sub>off</sub>)—are crucial in determining the overall affinity. Kd is related to these rate constants by the equation: Kd = k<sub>off</sub>/k<sub>on</sub>. A high k<sub>off</sub> or a low k<sub>on</sub> indicates weak binding. Studying these rate constants provides further insights into the mechanisms governing enzyme-substrate interactions.
Conclusion: A Fundamental Concept with Broad Implications
Enzyme binding affinity is a fundamental concept in biochemistry and enzymology. It governs the efficiency and specificity of enzyme-catalyzed reactions, influencing a wide array of biological processes and technological applications. A deep understanding of this concept, including its measurement and the factors that influence it, is vital for advancing our knowledge of biological systems and for developing novel therapeutic and industrial applications. Further research continues to uncover the intricate details of enzyme-substrate interactions, providing ever-more refined tools and insights into the fascinating world of enzymes. The ongoing exploration of enzyme binding affinity promises to yield significant advances in fields ranging from medicine to biotechnology.
Latest Posts
Latest Posts
-
What Is The Functional Unit Of Heredity
Mar 31, 2025
-
Cracking The Code Of Life Answer Key
Mar 31, 2025
-
Delta H Is Negative Exothermic Or Endothermic
Mar 31, 2025
-
Raising And Lowering Operators Angular Momentum
Mar 31, 2025
-
Unauthrized Data Rollbak Or Undo Cia Triad
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Does Enzyme Binding Affinity Mean . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
