What Does Kw Stand For In Chemistry
Muz Play
Mar 15, 2025 · 6 min read
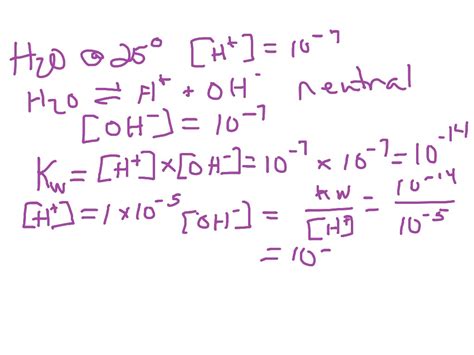
Table of Contents
What Does KW Stand For in Chemistry? Understanding the Ion Product Constant of Water
The abbreviation "Kw" in chemistry stands for the ion product constant of water. It's a crucial concept in understanding acid-base chemistry and aqueous solutions, representing the equilibrium constant for the autoionization of water. This article will delve deep into the meaning, significance, and applications of Kw, exploring its relationship with pH, temperature, and various chemical processes.
Understanding the Autoionization of Water
Water, while seemingly simple, undergoes a process called autoionization or self-ionization. This means that water molecules can spontaneously react with each other, transferring a proton (H⁺) from one molecule to another. This process is represented by the following equilibrium reaction:
2H₂O(l) ⇌ H₃O⁺(aq) + OH⁻(aq)
This equation shows that two water molecules react to form a hydronium ion (H₃O⁺) and a hydroxide ion (OH⁻). The hydronium ion is essentially a protonated water molecule, representing the presence of a proton in solution. The hydroxide ion is formed when a water molecule loses a proton.
This equilibrium is dynamic; water molecules are constantly ionizing and recombining. However, at any given moment, a small but significant concentration of both hydronium and hydroxide ions exists in pure water.
Defining the Ion Product Constant, Kw
The ion product constant of water (Kw) is the equilibrium constant for the autoionization reaction described above. It's defined as the product of the concentrations of hydronium and hydroxide ions:
Kw = [H₃O⁺][OH⁻]
At 25°C (298 K), the value of Kw is approximately 1.0 x 10⁻¹⁴. This means that in pure water at this temperature, the concentration of both hydronium and hydroxide ions is 1.0 x 10⁻⁷ M. It's crucial to remember that this value is temperature-dependent; Kw increases with increasing temperature.
Implications of Kw = 1.0 x 10⁻¹⁴
This seemingly small value of Kw has profound implications:
-
Neutrality of Pure Water: In pure water, the concentrations of H₃O⁺ and OH⁻ are equal, resulting in a neutral solution. This is because the value of Kw directly relates the concentration of H₃O⁺ and OH⁻. If one increases, the other must decrease to maintain the constant value of Kw.
-
Basis for pH Scale: The pH scale, a measure of the acidity or basicity of a solution, is directly related to the concentration of hydronium ions. The pH is defined as the negative logarithm (base 10) of the hydronium ion concentration:
pH = -log₁₀[H₃O⁺]Similarly, pOH is defined as:
pOH = -log₁₀[OH⁻]Since Kw = [H₃O⁺][OH⁻], we can derive the relationship:
pH + pOH = 14 (at 25°C)This equation is extremely useful for calculating the pH or pOH of a solution if one of them is known.
Temperature Dependence of Kw
It is vital to understand that Kw is not a constant across all temperatures. As temperature increases, the autoionization of water becomes more favorable, leading to a higher concentration of both H₃O⁺ and OH⁻ ions. Therefore, Kw increases with temperature. This means that the neutrality point (where [H₃O⁺] = [OH⁻]) is still maintained, but the actual concentrations are higher at elevated temperatures.
This temperature dependence highlights the importance of specifying the temperature when discussing the value of Kw. While 1.0 x 10⁻¹⁴ is a commonly used value, it's only accurate at 25°C. At higher temperatures, the value of Kw will be significantly larger.
Kw and Acid-Base Equilibria
Kw plays a central role in understanding acid-base equilibria in aqueous solutions. Acids increase the concentration of H₃O⁺ ions, while bases increase the concentration of OH⁻ ions. Knowing Kw allows us to calculate the concentrations of these ions in various solutions, regardless of whether the solution is acidic, basic, or neutral.
For example, consider a strong acid like HCl. When HCl dissolves in water, it completely dissociates, significantly increasing the concentration of H₃O⁺ ions. Using Kw, we can calculate the corresponding decrease in OH⁻ ion concentration to maintain the constant value of Kw at the given temperature.
Similarly, for a strong base like NaOH, the increase in OH⁻ concentration leads to a decrease in H₃O⁺ concentration, again maintaining the value of Kw.
Applications of Kw
The ion product constant of water has widespread applications in various fields:
-
Environmental Science: Kw is crucial for understanding water quality and determining the acidity or basicity of natural water bodies like lakes and rivers. Acid rain, for instance, lowers the pH of water, significantly affecting aquatic life.
-
Medicine and Biology: The pH of bodily fluids is carefully regulated to maintain homeostasis. Kw is essential for understanding the acid-base balance in blood and other biological systems. Many biochemical reactions are highly sensitive to pH changes.
-
Analytical Chemistry: Kw is used in numerous analytical techniques, such as titration, to calculate the concentrations of acids and bases in various solutions.
-
Industrial Processes: Many industrial processes involve aqueous solutions, and understanding Kw is crucial for optimizing these processes and ensuring product quality.
Beyond the Basics: Exploring More Complex Scenarios
While the basic concept of Kw is relatively straightforward, its applications can extend to more complex scenarios. For instance:
-
Weak Acids and Bases: Calculating the pH of weak acid or weak base solutions requires considering the equilibrium constant (Ka or Kb) alongside Kw. This involves solving equilibrium expressions which often require the use of approximations or iterative methods.
-
Buffers: Buffer solutions resist changes in pH upon the addition of small amounts of acid or base. The ability of a buffer to maintain a relatively constant pH is directly related to Kw and the equilibrium constants of the weak acid and its conjugate base (or weak base and its conjugate acid) that constitute the buffer.
-
Solubility Equilibria: The solubility of certain sparingly soluble salts is affected by the pH of the solution. Understanding Kw helps in predicting and controlling the solubility of these salts.
-
Non-Aqueous Solvents: While Kw is specifically defined for water, the concept of self-ionization and an analogous equilibrium constant can be extended to other solvents. However, the value of the equivalent constant will vary significantly depending on the solvent's properties.
Conclusion
The ion product constant of water (Kw) is a fundamental concept in chemistry, playing a pivotal role in understanding acid-base chemistry and aqueous solutions. Its value, while seemingly small, has profound implications for numerous aspects of science and technology. Understanding its temperature dependence and its relationship to pH is crucial for accurate calculations and interpretations in various fields, ranging from environmental science to medicine and industrial processes. While the basic definition is simple, the applications of Kw extend to complex scenarios requiring a deeper understanding of equilibrium chemistry. Mastering Kw is essential for any student or professional working with aqueous solutions and their chemical properties.
Latest Posts
Latest Posts
-
Energy Required To Remove An Electron From A Gaseous Atom
Mar 15, 2025
-
Que Es La Descomposicion De Acidos
Mar 15, 2025
-
Which Factor Affects Congressional Approval Ratings The Most
Mar 15, 2025
-
Fourier Transform Of A Differential Equation
Mar 15, 2025
-
Which One Neutral Charge Proton Or Neutron
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about What Does Kw Stand For In Chemistry . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
