What Happens To Electrons In A Covalent Bond
Muz Play
Mar 30, 2025 · 6 min read
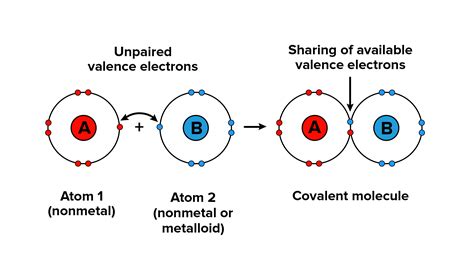
Table of Contents
What Happens to Electrons in a Covalent Bond? A Deep Dive into Sharing
Covalent bonds are the fundamental building blocks of many molecules, forming the backbone of organic chemistry and playing a crucial role in countless biological processes. Understanding what happens to electrons in a covalent bond is key to grasping the properties and behavior of these molecules. This article will delve into the intricacies of covalent bonding, exploring the electron sharing mechanisms, different types of covalent bonds, factors influencing bond strength, and the implications of covalent bonding in various contexts.
The Essence of Covalent Bonding: Sharing is Caring
Unlike ionic bonds, where electrons are transferred from one atom to another, covalent bonds involve the sharing of electrons between atoms. This sharing occurs when atoms approach each other closely enough that their valence electron orbitals overlap. This overlap creates a region of high electron density between the nuclei, which attracts both nuclei and holds the atoms together. The shared electrons are essentially attracted to both atomic nuclei simultaneously, creating a stable molecular structure.
The Role of Valence Electrons
The key players in covalent bond formation are the valence electrons. These are the electrons located in the outermost shell of an atom. Atoms strive to achieve a stable electron configuration, often resembling that of a noble gas (eight valence electrons, or a full octet). By sharing electrons, atoms can effectively complete their valence shells and achieve greater stability. This pursuit of stability is the driving force behind covalent bond formation.
Overlapping Atomic Orbitals
The process of sharing involves the overlap of atomic orbitals. These orbitals represent the regions of space where an electron is most likely to be found. When atomic orbitals overlap, the electrons can exist in the shared space between the nuclei, creating a molecular orbital. This molecular orbital is lower in energy than the individual atomic orbitals, resulting in a more stable system. The extent of overlap significantly impacts the strength of the covalent bond.
Types of Covalent Bonds: A Spectrum of Sharing
Covalent bonds aren't all created equal. The degree of electron sharing and the nature of the bond can vary, leading to different types of covalent bonds:
1. Nonpolar Covalent Bonds: Equal Sharing
In nonpolar covalent bonds, the electrons are shared equally between the two atoms. This occurs when the atoms involved have similar electronegativity. Electronegativity is a measure of an atom's ability to attract electrons in a chemical bond. If the electronegativity difference between two atoms is small (generally less than 0.4), the bond is considered nonpolar. Examples include the bonds in diatomic molecules like H₂, O₂, and Cl₂.
2. Polar Covalent Bonds: Unequal Sharing
In polar covalent bonds, the electrons are shared unequally. This happens when the atoms involved have different electronegativities. The atom with higher electronegativity attracts the shared electrons more strongly, creating a partial negative charge (δ-) on that atom and a partial positive charge (δ+) on the other atom. This results in a dipole moment, a measure of the polarity of the bond. Water (H₂O) is a classic example, with oxygen being more electronegative than hydrogen, leading to polar O-H bonds.
3. Coordinate Covalent Bonds: One Atom Provides Both Electrons
A coordinate covalent bond, also known as a dative bond, is a special type of covalent bond where one atom provides both electrons for the shared pair. This often occurs in molecules containing lone pairs of electrons, which can be donated to an atom that needs electrons to complete its octet. Examples are found in many complex ions and molecules.
Factors Influencing Covalent Bond Strength: A Tug-of-War
Several factors influence the strength of a covalent bond, which is directly related to the energy required to break the bond. Stronger bonds require more energy to break.
1. Atomic Size: Closer is Stronger
Smaller atoms generally form stronger covalent bonds because their valence electrons are closer to the nucleus and experience a stronger attraction. As atomic size increases, the distance between the nucleus and the valence electrons increases, weakening the bond.
2. Electronegativity: A Balancing Act
The electronegativity difference between atoms influences bond strength. While a large difference leads to ionic bonding, a moderate difference can result in stronger polar covalent bonds due to the stronger attraction between the partially charged atoms. However, excessively large electronegativity differences can weaken the bond by distorting the electron distribution.
3. Bond Order: Multiple Bonds, Stronger Bonds
The bond order indicates the number of electron pairs shared between two atoms. A single bond has a bond order of 1, a double bond has a bond order of 2, and a triple bond has a bond order of 3. Higher bond orders generally result in stronger bonds because more electrons are shared, leading to a greater attractive force between the atoms. For example, a triple bond (like in N₂) is stronger than a double bond (like in O₂), which is stronger than a single bond (like in Cl₂).
4. Resonance: Delocalization Strengthens Bonds
In some molecules, the electron density is delocalized over multiple atoms, a phenomenon known as resonance. This delocalization strengthens the bonds by spreading the electron density over a larger region, resulting in a more stable structure. Benzene is a classic example where resonance stabilizes the molecule and leads to stronger bonds than predicted by a single Lewis structure.
Implications of Covalent Bonding: A Wide-Ranging Impact
Covalent bonding plays a crucial role in determining the properties and behavior of molecules across various fields:
1. Organic Chemistry: The Foundation of Life
Covalent bonding is the cornerstone of organic chemistry, the study of carbon-containing compounds. Carbon's ability to form four covalent bonds allows it to create a vast array of complex molecules, forming the basis of all known life forms. From simple hydrocarbons to complex biomolecules like proteins and DNA, covalent bonds hold these structures together.
2. Biochemistry: Life's Processes
In biochemistry, covalent bonds are essential for the structure and function of biological molecules. Peptide bonds, which link amino acids in proteins, are covalent bonds. Glycosidic bonds, which link sugar units in carbohydrates, are also covalent. These bonds determine the three-dimensional structure and properties of these vital molecules.
3. Material Science: Designing New Materials
Understanding covalent bonding is crucial in material science for designing new materials with specific properties. The strength, hardness, and other properties of materials are directly related to the type and strength of the covalent bonds present. Researchers leverage this knowledge to develop advanced materials for various applications.
4. Environmental Science: Understanding Chemical Reactions
In environmental science, understanding covalent bonding is crucial for analyzing chemical reactions and their impact on the environment. The formation and breaking of covalent bonds are central to many environmental processes, including pollution, degradation, and biogeochemical cycles.
Conclusion: A Fundamental Force of Nature
Covalent bonding, with its intricate mechanisms of electron sharing, is a fundamental force shaping the world around us. From the molecules of life to advanced materials, understanding the behavior of electrons in covalent bonds is crucial for advancements across numerous scientific and technological disciplines. The concepts discussed here provide a solid foundation for further exploration into the fascinating world of chemical bonding. Further research into the intricacies of orbital overlap, bond polarization, and the influence of molecular geometry will deepen one's understanding of this essential aspect of chemistry. The continuous evolution of research in this field promises exciting new discoveries and applications in the years to come.
Latest Posts
Latest Posts
-
Cross Section Of A Woody Stem
Apr 01, 2025
-
A State Function Is Best Described As
Apr 01, 2025
-
What Is The Electron Configuration For Ne
Apr 01, 2025
-
Difference Between E1 And E2 Reaction
Apr 01, 2025
-
Journal Entry To Issue Common Stock
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Happens To Electrons In A Covalent Bond . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
