What Is A Change Of State
Muz Play
Mar 21, 2025 · 6 min read
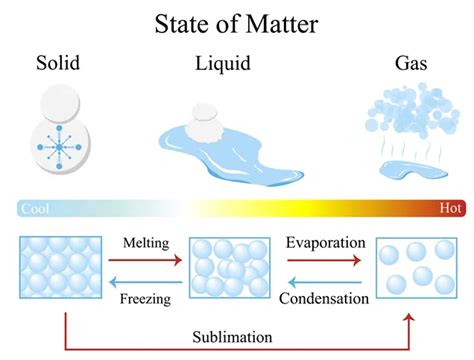
Table of Contents
- What Is A Change Of State
- Table of Contents
- What is a Change of State? A Comprehensive Guide
- The States of Matter: A Quick Recap
- Types of Changes of State
- Factors Affecting Changes of State
- Applications of Changes of State
- Understanding Phase Diagrams
- Advanced Concepts and Further Exploration
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
What is a Change of State? A Comprehensive Guide
Changes of state, also known as phase transitions, are fundamental processes in physics and chemistry that describe the transformation of a substance from one state of matter to another. Understanding these changes is crucial across various scientific disciplines, from meteorology predicting weather patterns to materials science developing new technologies. This comprehensive guide will explore the different states of matter, the mechanisms driving changes of state, and the practical applications of this knowledge.
The States of Matter: A Quick Recap
Before diving into the transitions themselves, let's briefly review the common states of matter:
-
Solid: A solid possesses a definite shape and volume. Its particles are tightly packed in a fixed arrangement, resulting in strong intermolecular forces. This leads to rigidity and resistance to changes in shape or volume. Examples include ice, rock, and wood.
-
Liquid: A liquid has a definite volume but takes the shape of its container. Its particles are closer together than in a gas but are not rigidly arranged, allowing them to move past one another. The intermolecular forces are weaker than in solids, resulting in fluidity. Examples include water, oil, and mercury.
-
Gas: A gas has neither a definite shape nor volume; it expands to fill the available space. Its particles are widely dispersed and move independently, with weak intermolecular forces. This results in compressibility and the ability to expand. Examples include air, oxygen, and carbon dioxide.
-
Plasma: Often considered the fourth state of matter, plasma is an ionized gas. It consists of freely moving ions and electrons, resulting in a highly conductive state. Plasmas are found in stars, lightning, and fluorescent lights.
-
Bose-Einstein Condensate (BEC): This exotic state of matter occurs at extremely low temperatures, where atoms behave as a single quantum entity. BECs exhibit unique properties and are a subject of ongoing research.
Types of Changes of State
Changes of state are driven by the addition or removal of energy, typically in the form of heat. There are six primary types:
-
Melting (Solid to Liquid): This occurs when a solid absorbs enough heat to overcome the intermolecular forces holding its particles together. The particles gain kinetic energy, allowing them to move more freely and transition to the liquid state. The temperature at which melting occurs is called the melting point.
-
Freezing (Liquid to Solid): The opposite of melting, freezing occurs when a liquid loses heat, causing its particles to slow down and lose kinetic energy. The intermolecular forces become strong enough to hold the particles in a fixed arrangement, forming a solid. The temperature at which freezing occurs is called the freezing point. Note that for most substances, the melting and freezing points are the same.
-
Vaporization (Liquid to Gas): Vaporization is the process by which a liquid transforms into a gas. This can occur in two ways:
-
Boiling: Boiling occurs when a liquid is heated to its boiling point, the temperature at which the vapor pressure equals atmospheric pressure. Bubbles of vapor form throughout the liquid and rise to the surface.
-
Evaporation: Evaporation occurs at temperatures below the boiling point. It is a surface phenomenon where liquid molecules with sufficient kinetic energy escape the liquid's surface and enter the gaseous phase.
-
-
Condensation (Gas to Liquid): Condensation is the opposite of vaporization. It occurs when a gas loses heat, causing its particles to slow down and lose kinetic energy. The intermolecular forces become strong enough to hold the particles together, forming a liquid. This is often observed as dew formation or the condensation of water vapor on a cold surface.
-
Sublimation (Solid to Gas): Sublimation is the transition from a solid directly to a gas without passing through the liquid phase. This occurs when the vapor pressure of the solid exceeds atmospheric pressure at a temperature below the melting point. Dry ice (solid carbon dioxide) is a common example.
-
Deposition (Gas to Solid): Deposition is the opposite of sublimation, involving the direct transition from a gas to a solid without passing through the liquid phase. Frost formation is a prime example of deposition.
Factors Affecting Changes of State
Several factors influence the rate and temperature at which changes of state occur:
-
Temperature: Higher temperatures generally increase the kinetic energy of particles, favoring transitions to less ordered states (solid to liquid, liquid to gas). Lower temperatures have the opposite effect.
-
Pressure: Increased pressure generally favors more compact states of matter. Higher pressure can increase the boiling point of a liquid and decrease the melting point of a solid. Conversely, lower pressure generally lowers the boiling point.
-
Intermolecular Forces: Stronger intermolecular forces require more energy to overcome, resulting in higher melting and boiling points.
-
Impurities: The presence of impurities can affect the melting and boiling points of a substance. For example, adding salt to water raises its boiling point and lowers its freezing point.
Applications of Changes of State
Changes of state are vital in numerous applications:
-
Weather: The water cycle, involving evaporation, condensation, and precipitation, is a crucial aspect of weather patterns.
-
Refrigeration and Air Conditioning: These systems utilize the vaporization and condensation of refrigerants to transfer heat and cool spaces.
-
Industrial Processes: Many industrial processes, such as distillation and crystallization, rely on changes of state for purification and separation of materials.
-
Material Science: Understanding changes of state is critical in designing and processing materials with specific properties.
-
Food Preservation: Freezing and drying are common methods of food preservation that rely on changes of state.
-
Medical Applications: Cryosurgery, which uses extremely low temperatures to destroy abnormal tissue, utilizes the freezing of biological materials.
Understanding Phase Diagrams
Phase diagrams are graphical representations that illustrate the conditions (temperature and pressure) under which different phases of a substance exist. They are extremely useful for visualizing the transitions between states. A typical phase diagram shows regions representing solid, liquid, and gaseous phases, along with lines indicating the conditions at which phase transitions occur (e.g., melting/freezing, boiling/condensation). Triple points, where all three phases coexist in equilibrium, are also highlighted. Understanding phase diagrams allows for predicting the state of a substance under various conditions.
Advanced Concepts and Further Exploration
The study of changes of state extends beyond the basic concepts outlined above. More advanced topics include:
-
Critical Point: The temperature and pressure above which the distinction between liquid and gas disappears.
-
Supercritical Fluids: Fluids existing beyond the critical point, possessing unique properties that find applications in various fields.
-
Metastable States: States that are not thermodynamically stable but persist for a certain time. Examples include supercooled liquids and supersaturated solutions.
-
Thermodynamics of Phase Transitions: A deeper dive into the energetic considerations involved in phase transitions, utilizing concepts like Gibbs free energy and enthalpy.
Conclusion
Changes of state are fundamental processes with far-reaching implications across numerous scientific and technological disciplines. Understanding the mechanisms driving these transitions, the factors influencing them, and their applications is crucial for advancements in various fields. From predicting weather patterns to developing new materials and technologies, the knowledge of changes of state is invaluable. This comprehensive guide provides a solid foundation for further exploration of this fascinating area of science. Further research into the advanced concepts mentioned can deepen your understanding of the intricate world of phase transitions.
Latest Posts
Latest Posts
-
What Is Ac Voltage And Dc Voltage
Mar 23, 2025
-
What Is The Difference Between Products And Reactants
Mar 23, 2025
-
Chemical Reaction Of Metals With Bases
Mar 23, 2025
-
First Order Partial Differential Equations Examples
Mar 23, 2025
-
How To Find Mass Of Excess Reactant
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about What Is A Change Of State . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
