What Is A Family On The Periodic Table Of Elements
Muz Play
Mar 16, 2025 · 6 min read
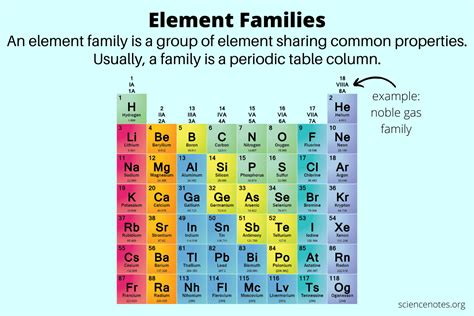
Table of Contents
What is a Family on the Periodic Table of Elements? Understanding Groups and Their Properties
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and recurring properties. While periods represent horizontal rows indicating energy levels, families, also known as groups, are the vertical columns that showcase elements with similar chemical behaviors. Understanding families is crucial to predicting element properties and understanding chemical reactions. This article delves deep into the concept of families on the periodic table, exploring their characteristics, identifying key families, and highlighting the importance of their periodic arrangement.
The Defining Feature: Valence Electrons
The fundamental reason elements within a family share similar properties lies in their valence electrons. These are the electrons in the outermost shell of an atom, actively participating in chemical bonding. Elements in the same family possess the same number of valence electrons, leading to predictable reactivity patterns. For example, alkali metals (Group 1) all have one valence electron, making them highly reactive and prone to losing that electron to achieve a stable electron configuration.
Predicting Reactivity Based on Valence Electrons
The number of valence electrons directly influences an element's reactivity. Elements strive to achieve a stable electron configuration, often resembling that of a noble gas (Group 18). This tendency drives chemical bonding. Elements with nearly full valence shells (like halogens in Group 17) readily gain electrons, while elements with few valence electrons (like alkali metals) readily lose them. Understanding this fundamental principle allows chemists to predict how elements will interact and form compounds.
Key Families on the Periodic Table: A Closer Look
The periodic table is structured with 18 groups, each representing a distinct family. Let's examine some of the most prominent families:
1. Alkali Metals (Group 1)
- Defining characteristic: One valence electron.
- Reactivity: Extremely reactive, readily losing their valence electron to form +1 ions. They react violently with water, producing hydrogen gas.
- Examples: Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Rb), Cesium (Cs), Francium (Fr).
- Physical properties: Soft, silvery-white metals with low densities and melting points.
2. Alkaline Earth Metals (Group 2)
- Defining characteristic: Two valence electrons.
- Reactivity: Reactive, but less so than alkali metals. They lose their two valence electrons to form +2 ions.
- Examples: Beryllium (Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Ba), Radium (Ra).
- Physical properties: Harder, denser, and have higher melting points than alkali metals.
3. Transition Metals (Groups 3-12)
- Defining characteristic: Variable number of valence electrons, involving both s and d orbitals.
- Reactivity: Varying reactivity, often forming multiple oxidation states. Known for their catalytic properties.
- Examples: Iron (Fe), Copper (Cu), Zinc (Zn), Gold (Au), Platinum (Pt).
- Physical properties: Typically hard, dense, and have high melting and boiling points. Many are excellent conductors of heat and electricity.
4. Halogens (Group 17)
- Defining characteristic: Seven valence electrons.
- Reactivity: Highly reactive nonmetals, readily gaining one electron to form -1 ions. They exist as diatomic molecules (e.g., Cl2, Br2).
- Examples: Fluorine (F), Chlorine (Cl), Bromine (Br), Iodine (I), Astatine (At).
- Physical properties: Their states vary from gas (F2, Cl2) to liquid (Br2) to solid (I2).
5. Noble Gases (Group 18)
- Defining characteristic: Eight valence electrons (except helium, which has two).
- Reactivity: Extremely unreactive, often called inert gases due to their stable electron configurations.
- Examples: Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe), Radon (Rn).
- Physical properties: Colorless, odorless, monatomic gases.
6. Rare Earth Elements (Lanthanides and Actinides)
- Defining characteristic: These elements are found separately at the bottom of the periodic table. They are characterized by the filling of the 4f and 5f orbitals respectively.
- Reactivity: Reactivity varies significantly within the group.
- Examples: Lanthanides: Cerium (Ce), Praseodymium (Pr), Neodymium (Nd), etc. Actinides: Uranium (U), Plutonium (Pu), etc.
- Physical properties: Many are radioactive. Their properties are more complex and nuanced than those in the main body of the periodic table.
Beyond the Basic Families: Understanding Subgroups and Trends
While the main group families provide a solid foundation, the periodic table's intricacies extend beyond these classifications. Understanding subgroups and trends within families is essential for a comprehensive grasp of elemental behavior.
Subgroups and Anomalies
Some families are further divided into subgroups, reflecting subtle variations in properties. For example, within the transition metals, subgroups exist based on the filling of d orbitals. However, it's crucial to acknowledge that there are anomalies. Not every element perfectly fits the characteristics of its family. Factors like atomic size, electronegativity, and ionization energy can introduce variations in behavior.
Periodic Trends: Atomic Radius, Ionization Energy, and Electronegativity
Understanding periodic trends is critical to predicting element behavior. These trends, which exhibit patterns across the periodic table, include:
- Atomic Radius: Generally increases down a group and decreases across a period.
- Ionization Energy: The energy required to remove an electron. It generally decreases down a group and increases across a period.
- Electronegativity: The ability of an atom to attract electrons in a chemical bond. It generally increases across a period and decreases down a group.
These trends are directly linked to the arrangement of electrons in atoms and their interactions. They offer valuable insights into chemical bonding and reactivity.
The Importance of Family in Chemical Reactions
The concept of elemental families is not just a classificatory tool; it's a powerful predictive instrument in chemistry. Understanding the properties of a family allows us to:
- Predict reaction products: Knowing the typical reactivity of a family helps us anticipate the outcome of chemical reactions involving its members.
- Design new materials: The properties of elements within a family allow for the design of materials with specific characteristics.
- Understand biological processes: Many biological processes are intricately linked to the properties of specific elemental families. For example, the role of alkali metals in nerve impulse transmission or the importance of halogens in various biological molecules.
Applications Across Diverse Fields
The understanding of elemental families extends far beyond the realm of theoretical chemistry. It finds practical applications in various fields, including:
- Materials Science: The unique properties of different families are crucial in the development of advanced materials like alloys, semiconductors, and catalysts.
- Medicine: Elements from various families play crucial roles in pharmaceuticals, medical imaging techniques, and radiation therapy.
- Environmental Science: Understanding the chemical behavior of elements allows for the development of strategies for pollution control and environmental remediation.
- Energy Production: The properties of specific families are key to advancements in renewable energy technologies.
Conclusion: A Foundation for Chemical Understanding
Families on the periodic table are not simply arbitrary groupings; they represent a fundamental organization based on shared electron configurations and resulting properties. By understanding the characteristics of different families and the periodic trends that govern their behavior, we gain a powerful predictive tool for exploring chemical reactions, designing new materials, and advancing our understanding of the natural world. The arrangement of elements into families is a testament to the power of scientific organization and a cornerstone of modern chemistry, continuously enriching our understanding of matter and its interactions. Further exploration of specific families and their intricate properties will reveal even more profound insights into the fascinating world of chemistry.
Latest Posts
Latest Posts
-
Can A Buffer Be Made With A Strong Acid
Mar 17, 2025
-
Gas Laws Practice Problems With Answers
Mar 17, 2025
-
Are Strong Bases Good Leaving Groups
Mar 17, 2025
-
Which Polymer Is Composed Of Amino Acids
Mar 17, 2025
-
According To Dalton Atoms Of Different Elements Will Be
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Is A Family On The Periodic Table Of Elements . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
