What Is A Kirby Bauer Test
Muz Play
Mar 23, 2025 · 7 min read
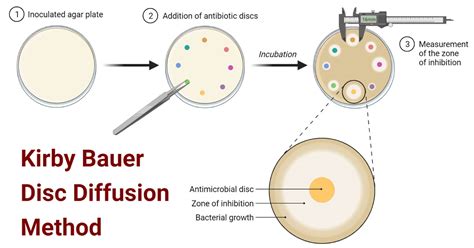
Table of Contents
What is a Kirby-Bauer Test? Your Comprehensive Guide
The Kirby-Bauer test, also known as the disk diffusion test, is a crucial microbiological technique used to determine the susceptibility of bacteria to various antimicrobial agents. This simple yet powerful method provides essential information for guiding antibiotic treatment decisions in clinical settings. Understanding its principles, procedure, and interpretation is fundamental for anyone working in microbiology, infectious disease management, or related fields. This comprehensive guide delves into every aspect of the Kirby-Bauer test, equipping you with the knowledge to appreciate its significance and limitations.
Understanding the Principles of the Kirby-Bauer Test
At its core, the Kirby-Bauer test assesses the effectiveness of antimicrobial agents against specific bacterial strains by observing their inhibitory effects on bacterial growth. The principle relies on the diffusion of the antimicrobial agent from a filter paper disk placed on an agar plate inoculated with the bacterial culture.
Diffusion and Inhibition Zones: The Key to Interpretation
The antimicrobial agent diffuses outwards from the disk into the surrounding agar. If the bacteria are susceptible to the agent, a clear zone of inhibition will develop around the disk, where bacterial growth is prevented. The size of this zone of inhibition is directly correlated with the degree of susceptibility. A larger zone indicates greater susceptibility, while a smaller or absent zone suggests resistance. The diameter of the zone of inhibition is measured in millimeters and compared to established interpretive standards to classify the bacterial strain as susceptible, intermediate, or resistant.
Factors Influencing Zone of Inhibition Size
Several factors influence the size of the zone of inhibition, including:
- Antimicrobial agent concentration: Higher concentrations lead to larger zones of inhibition.
- Antimicrobial agent diffusion rate: Agents with higher diffusion rates produce larger zones.
- Bacterial inoculum size: A larger inoculum results in smaller zones.
- Growth rate of the bacteria: Fast-growing bacteria may show smaller zones.
- Agar depth: The depth of the agar influences diffusion, impacting zone size.
- Incubation time and temperature: These factors affect bacterial growth and antimicrobial activity.
Step-by-Step Procedure of the Kirby-Bauer Test
The Kirby-Bauer test is performed using a standardized procedure to ensure reliable and comparable results. Deviation from the established protocol can significantly impact the accuracy of the test.
1. Preparation of Bacterial Inoculum
A pure bacterial culture is required. The bacteria are grown in broth culture to a standardized turbidity, typically matching a 0.5 McFarland standard. This ensures a consistent bacterial concentration for all tests. The turbidity is checked using a McFarland turbidimeter or by visual comparison to a McFarland standard.
2. Inoculation of Agar Plates
A sterile cotton swab is dipped into the standardized bacterial suspension and used to inoculate the surface of a Mueller-Hinton agar plate. The plate is evenly inoculated by streaking the swab across the entire surface in three directions, rotating the plate by 60 degrees between each streak. The inoculated plate is left to dry for several minutes to allow for even distribution of bacteria.
3. Application of Antimicrobial Disks
Pre-prepared antimicrobial disks containing known concentrations of different antibiotics are placed onto the inoculated agar surface using sterile forceps. The disks are pressed gently to ensure good contact with the agar. Each plate typically contains several disks, testing different antibiotics. The disks are spaced according to the standardized guidelines to prevent overlapping zones of inhibition.
4. Incubation
The inoculated plates are inverted and incubated at 35°C for 16-18 hours. Incubation at the correct temperature and for the appropriate duration is critical for accurate results.
5. Measurement and Interpretation of Zones of Inhibition
After incubation, the diameter of the zone of inhibition around each disk is measured in millimeters using a ruler. The measurement is made perpendicular to the direction of the disk. These measurements are compared to standardized interpretive charts provided by the Clinical and Laboratory Standards Institute (CLSI) or similar organizations. These charts specify the zone diameter thresholds that classify bacteria as susceptible, intermediate, or resistant to the particular antimicrobial agent.
Interpretation of Results: Susceptible, Intermediate, or Resistant
The interpretive standards provided by CLSI are crucial for accurate interpretation. The size of the zone of inhibition is compared to these standards for each antibiotic tested.
- Susceptible (S): The bacteria are inhibited by the antibiotic at clinically achievable concentrations. Treatment with this antibiotic is likely to be effective.
- Intermediate (I): The bacteria exhibit intermediate susceptibility. The outcome of treatment with this antibiotic is uncertain. Factors such as the site of infection and the concentration of the antibiotic achievable at the site may influence treatment success.
- Resistant (R): The bacteria are not inhibited by the antibiotic at clinically achievable concentrations. Treatment with this antibiotic is unlikely to be effective.
Limitations of the Kirby-Bauer Test
While the Kirby-Bauer test is a valuable tool, it has certain limitations:
- It only measures the in vitro activity of the antimicrobial agent. The in vivo response of the bacteria to treatment may differ due to host factors such as immune response and drug distribution.
- It doesn't provide information about the Minimum Inhibitory Concentration (MIC). MIC is the lowest concentration of antibiotic that inhibits bacterial growth. While not directly measured, MIC can be inferred by comparing the zone size to MIC breakpoints from CLSI guidelines.
- It may not accurately predict the outcome of treatment for all types of infections. Certain infections may require different approaches to treatment, even if the bacteria are susceptible in vitro.
- It is not suitable for all bacteria or antimicrobial agents. Some bacteria may not grow well on Mueller-Hinton agar, and certain antimicrobial agents may not diffuse properly.
- Technical errors can influence results. Inaccurate inoculum preparation, improper disk placement, or incorrect incubation conditions can lead to unreliable results.
Applications of the Kirby-Bauer Test
The Kirby-Bauer test plays a vital role in various aspects of clinical microbiology and infectious disease management:
- Guiding antibiotic therapy: It is the primary method for determining the susceptibility of bacteria to various antibiotics, guiding clinical treatment decisions.
- Surveillance of antibiotic resistance: It provides valuable data for tracking the emergence and spread of antibiotic resistance in bacterial populations.
- Quality control of antibiotics: It can be used to assess the potency of antimicrobial agents.
- Research and development of new antibiotics: It helps evaluate the effectiveness of new antimicrobial agents against bacterial strains.
- Epidemiology of infectious diseases: It aids in studying the prevalence of antibiotic resistance in different regions and populations.
Ensuring Accurate Results: Quality Control and Standardization
Maintaining high standards throughout the Kirby-Bauer test procedure is essential for obtaining reliable and accurate results. This includes using standardized reagents, adhering to established protocols, and performing regular quality control checks.
Quality Control Measures:
- Using standardized reagents: Employing quality-assured Mueller-Hinton agar and antimicrobial disks is crucial. The composition of the agar must be within the specified ranges to allow for appropriate diffusion.
- Maintaining proper incubation conditions: Strict adherence to the specified temperature and incubation time is critical.
- Using a standardized inoculum: Preparing the bacterial inoculum to the correct turbidity is crucial for reproducible results.
- Regular quality control testing: Performing quality control tests using known bacterial strains with established susceptibility patterns helps to ensure the reliability of the test.
Beyond the Basics: Advanced Techniques and Considerations
While the standard Kirby-Bauer method is widely used, various advanced techniques and considerations are relevant:
- E-test: This method utilizes strips containing a gradient of antibiotic concentrations, providing a more precise determination of MIC.
- Automated systems: Automated systems can streamline the process, reducing manual handling and improving efficiency.
- Considering the Minimum Bactericidal Concentration (MBC): While the Kirby-Bauer test focuses on MIC, determining the MBC (the lowest concentration of antibiotic that kills bacteria) might provide additional information.
- Considering bacterial interactions: Some bacteria exhibit synergistic or antagonistic effects when exposed to multiple antibiotics simultaneously. This interaction is not always reflected in the individual zones of inhibition.
- Understanding the impact of pharmacokinetic and pharmacodynamic parameters: The effectiveness of antibiotics can be influenced by their pharmacokinetic and pharmacodynamic properties in the host.
Conclusion: The Enduring Importance of the Kirby-Bauer Test
The Kirby-Bauer test remains a cornerstone of clinical microbiology. Its simplicity, cost-effectiveness, and reliability make it an invaluable tool in the fight against infectious diseases. While limitations exist, proper execution and interpretation, along with consideration of other factors, provide crucial insights to inform appropriate antibiotic treatment strategies and contribute to global efforts in combating antibiotic resistance. Understanding the intricacies of this method is not just for microbiologists; it is vital for all healthcare professionals involved in the diagnosis and treatment of bacterial infections. The continuing relevance of the Kirby-Bauer test highlights its indispensable role in maintaining public health.
Latest Posts
Latest Posts
-
First Formulation Of The Categorical Imperative
Mar 25, 2025
-
Rearrangement Of Benzil To Benzilic Acid
Mar 25, 2025
-
Is Table Salt A Mixture Or Pure Substance
Mar 25, 2025
-
How To Do Inverse Laplace Transforms
Mar 25, 2025
-
Real World Application Of A Linear Equation In 2 Variables
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about What Is A Kirby Bauer Test . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
