What Is Lone Pairs In Chemistry
Muz Play
Mar 25, 2025 · 6 min read
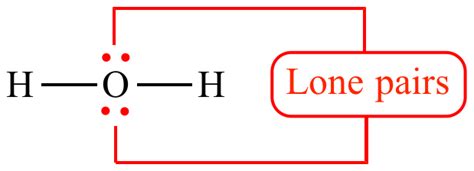
Table of Contents
What are Lone Pairs in Chemistry? A Deep Dive
Lone pairs, also known as unshared pairs or non-bonding pairs of electrons, are a fundamental concept in chemistry. Understanding them is crucial for predicting molecular geometry, reactivity, and various other properties of chemical compounds. This in-depth article will explore lone pairs, their impact on molecular structure, and their role in chemical reactions. We'll delve into the intricacies of VSEPR theory, hybridization, and the relationship between lone pairs and polarity.
Understanding the Basics: What are Lone Pairs?
In simple terms, a lone pair is a pair of valence electrons that are not involved in bonding with other atoms. Valence electrons are the outermost electrons of an atom, which participate in chemical bonding. Atoms strive to achieve a stable electron configuration, often resembling that of a noble gas (eight valence electrons – the octet rule, although there are exceptions). When an atom forms bonds, it shares its valence electrons with other atoms. However, some valence electrons remain unshared, forming a lone pair.
Example: Consider the water molecule (H₂O). Oxygen has six valence electrons. Two of these electrons form single bonds with two hydrogen atoms. The remaining four electrons form two lone pairs on the oxygen atom.
The Role of Lone Pairs in VSEPR Theory
The Valence Shell Electron Pair Repulsion (VSEPR) theory is a powerful model used to predict the three-dimensional shapes of molecules. This theory postulates that electron pairs (both bonding and lone pairs) repel each other and will arrange themselves to minimize this repulsion. Lone pairs exert a stronger repulsive force than bonding pairs, leading to distortions in molecular geometry.
VSEPR Theory and Molecular Geometry:
The arrangement of electron pairs (bonding and lone pairs) determines the electron-pair geometry, while the arrangement of only the atoms determines the molecular geometry. The presence of lone pairs significantly influences the molecular geometry, often resulting in deviations from ideal geometries.
Let's look at some examples:
-
Methane (CH₄): Carbon has four bonding pairs and no lone pairs. The electron-pair geometry and the molecular geometry are both tetrahedral.
-
Ammonia (NH₃): Nitrogen has three bonding pairs and one lone pair. The electron-pair geometry is tetrahedral, but the lone pair causes a distortion, resulting in a trigonal pyramidal molecular geometry. The lone pair pushes the bonding pairs closer together.
-
Water (H₂O): Oxygen has two bonding pairs and two lone pairs. The electron-pair geometry is tetrahedral, but the two lone pairs cause significant distortion, leading to a bent or V-shaped molecular geometry. The lone pairs repel each other strongly, pushing the hydrogen atoms closer together.
Lone Pairs and Hybridization
Hybridization is a concept that explains the bonding in molecules by mixing atomic orbitals to form hybrid orbitals. Lone pairs also participate in hybridization, influencing the type of hybrid orbitals formed.
Examples of Hybridization and Lone Pairs:
-
sp³ hybridization: This hybridization involves one s orbital and three p orbitals, resulting in four sp³ hybrid orbitals. In molecules like methane (CH₄), all four sp³ orbitals are used for bonding. In ammonia (NH₃) and water (H₂O), some sp³ hybrid orbitals accommodate lone pairs.
-
sp² hybridization: This involves one s orbital and two p orbitals, forming three sp² hybrid orbitals. One p orbital remains unhybridized. Molecules with sp² hybridization and lone pairs, such as formaldehyde (H₂CO), exhibit a trigonal planar electron pair geometry but a bent molecular geometry due to the presence of a lone pair on the oxygen atom.
-
sp hybridization: This involves one s orbital and one p orbital, forming two sp hybrid orbitals. Two p orbitals remain unhybridized. Molecules exhibiting sp hybridization and lone pairs, like hydrogen cyanide (HCN), have linear electron pair geometry and linear molecular geometry.
Lone Pairs and Molecular Polarity
Molecular polarity refers to the uneven distribution of electron density within a molecule. Lone pairs significantly contribute to molecular polarity.
A polar molecule has a net dipole moment, meaning it has a positive and a negative end. This occurs when there's a difference in electronegativity between the atoms in the molecule and an asymmetrical distribution of electrons, often influenced by lone pairs.
Examples:
-
Water (H₂O): The oxygen atom is more electronegative than the hydrogen atoms. The lone pairs on the oxygen atom contribute to an uneven distribution of electron density, making water a polar molecule.
-
Ammonia (NH₃): Similar to water, the nitrogen atom is more electronegative than the hydrogen atoms, and the lone pair contributes to the molecule's polarity.
-
Methane (CH₄): Despite the difference in electronegativity between carbon and hydrogen, the symmetrical tetrahedral structure cancels out the individual bond dipoles, making methane a nonpolar molecule.
Lone Pairs and Chemical Reactivity
Lone pairs play a vital role in chemical reactions. They act as electron donors, participating in reactions like Lewis acid-base reactions. A Lewis base is a molecule that donates an electron pair, and lone pairs are readily available for this donation.
Examples of Lone Pair Participation in Reactions:
-
Nucleophilic attack: Lone pairs can attack electron-deficient centers (electrophiles) in a reaction called a nucleophilic attack. This is a common mechanism in organic chemistry.
-
Coordination complexes: Lone pairs on ligands (molecules or ions) can donate electrons to a metal ion, forming coordination complexes. This is a cornerstone of coordination chemistry.
-
Hydrogen bonding: Lone pairs on highly electronegative atoms (like oxygen, nitrogen, and fluorine) can participate in hydrogen bonding, a relatively strong intermolecular force that significantly impacts the physical properties of many compounds.
Exceptions to the Octet Rule and Lone Pairs
While the octet rule is a useful guideline, many exceptions exist. Molecules with an expanded octet (more than eight valence electrons) or an incomplete octet (fewer than eight valence electrons) are common. Lone pairs still play a role in these exceptions, influencing the molecule's geometry and reactivity.
Examples of Exceptions:
-
Phosphorus pentachloride (PCl₅): Phosphorus has five bonding pairs and no lone pairs, expanding its octet to ten electrons.
-
Sulfur hexafluoride (SF₆): Sulfur has six bonding pairs and no lone pairs, expanding its octet to twelve electrons.
-
Boron trifluoride (BF₃): Boron has only three bonding pairs and no lone pairs, having an incomplete octet of six electrons.
Advanced Concepts: Lone Pair Stereochemistry and the Bent's Rule
The influence of lone pairs goes beyond basic VSEPR predictions. More advanced concepts like Bent's rule and the consideration of lone pair stereochemistry provide a deeper understanding of molecular properties.
Bent's rule suggests that more electronegative ligands preferentially occupy hybrid orbitals with a higher s-character. This can lead to further distortions in molecular geometries, especially when lone pairs are present. Understanding this subtle interplay of electronegativity and hybridization is crucial for accurate prediction of molecular structure and properties.
Lone pair stereochemistry also considers the spatial arrangement and interactions of lone pairs themselves. Their influence extends beyond simple repulsion, affecting factors like bond angles and molecular reactivity.
Conclusion: The Ubiquitous Lone Pair
Lone pairs are not mere afterthoughts in the world of chemistry; they are crucial players that significantly influence a molecule's structure, polarity, reactivity, and other properties. From predicting the shape of a water molecule to understanding the mechanisms of complex chemical reactions, appreciating the role of lone pairs is essential for a comprehensive understanding of chemistry. This detailed exploration has touched upon various aspects of lone pairs, ranging from basic VSEPR theory to more advanced concepts. By mastering this fundamental concept, you will gain a much stronger foundation in chemistry and be better equipped to tackle more advanced topics. The impact of lone pairs extends far beyond simple molecular geometry, influencing the entire chemical behavior of numerous substances. A deep understanding of lone pairs is, therefore, an indispensable part of any chemist's toolkit.
Latest Posts
Latest Posts
-
Anything That Has Mass And Volume Is Called
Mar 26, 2025
-
The Diels Alder Reaction Is A Concerted Reaction Define Concerted
Mar 26, 2025
-
How Many Fatty Acids Are Needed To Form A Glycerophospholipid
Mar 26, 2025
-
What Type Of Distortion Does The Good Homolosine Preserve
Mar 26, 2025
-
Autotrophs Make Their Own Food Using Energy From
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Is Lone Pairs In Chemistry . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
