What Is Primary Standard In Chemistry
Muz Play
Apr 01, 2025 · 6 min read
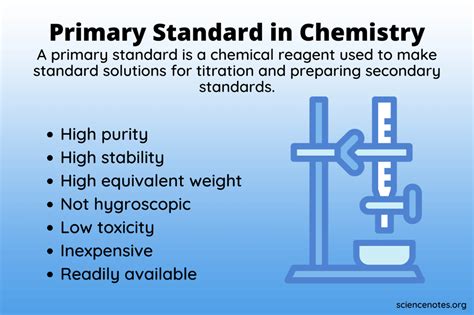
Table of Contents
What is a Primary Standard in Chemistry? A Comprehensive Guide
In the world of analytical chemistry, accuracy is paramount. When performing titrations or other quantitative analyses, the reliability of your results hinges on the accuracy of your reagents. This is where the concept of a primary standard comes into play. Understanding what constitutes a primary standard and its crucial role in ensuring accurate and precise chemical analysis is essential for any chemist, whether a seasoned professional or a budding student. This comprehensive guide will delve deep into the definition, characteristics, and applications of primary standards in chemistry.
Defining a Primary Standard
A primary standard is a highly purified chemical compound that serves as a highly accurate reference material for chemical analysis. It's used to standardize solutions (i.e., determine their exact concentration) before using them in titrations or other quantitative experiments. The reliability of the analysis directly depends on the purity and stability of the primary standard. Unlike secondary standards, which derive their concentration from a primary standard, primary standards have their concentration determined directly through stoichiometric calculations.
Key Characteristics of a Primary Standard
Not just any chemical compound can qualify as a primary standard. Several strict criteria must be met:
1. High Purity:
This is arguably the most crucial characteristic. The purity of a primary standard should be at least 99.9%, ideally even higher. Any impurities present will introduce errors in the calculations. The purity should be verified through various techniques, including spectroscopic and chromatographic methods.
2. Stability:
A primary standard must be stable under normal storage conditions. It should not react with atmospheric gases such as oxygen or carbon dioxide, nor should it readily decompose or absorb moisture. This stability ensures the long-term reliability of its concentration. Consider, for instance, how the deliquescent nature of NaOH prevents it from being a suitable primary standard.
3. High Molar Mass:
A high molar mass minimizes the effect of weighing errors on the overall accuracy of the analysis. A small error in weighing a substance with a high molar mass will result in a smaller percentage error in the calculated concentration compared to a substance with a low molar mass.
4. Easy to Dry and Handle:
The primary standard should be easily dried to remove any adsorbed water. It should also be non-hygroscopic to prevent the uptake of moisture from the atmosphere after drying. Furthermore, it should be easy to handle and weigh accurately without causing any contamination.
5. Readily Available:
A good primary standard should be readily available and relatively inexpensive. While purity is paramount, accessibility plays a crucial role in practical applications.
6. Defined Stoichiometry:
The primary standard should react completely and in a known stoichiometric ratio with the analyte (the substance being analyzed). This ensures accurate calculation of the concentration of the unknown solution.
Common Examples of Primary Standards
Several chemicals commonly serve as primary standards due to their excellent fulfillment of the criteria mentioned above. These include:
-
Potassium Hydrogen Phthalate (KHP): Often used to standardize solutions of strong bases like sodium hydroxide (NaOH). KHP is a monoprotic acid, meaning it donates only one proton per molecule in a reaction, making stoichiometric calculations straightforward. Its stability and ease of purification make it an ideal choice.
-
Sodium Carbonate (Na₂CO₃): Commonly employed to standardize solutions of strong acids like hydrochloric acid (HCl) or sulfuric acid (H₂SO₄). Its high molar mass helps minimize weighing errors. However, careful drying is crucial to eliminate adsorbed water.
-
Benzoic Acid (C₇H₆O₂): Another excellent primary standard for standardizing strong bases. It's a crystalline solid, relatively easy to purify, and stable under normal conditions.
-
Potassium Dichromate (K₂Cr₂O₇): Frequently used in redox titrations. It is a strong oxidizing agent with a stable, well-defined stoichiometry, making it suitable for standardizing reducing agents.
-
Sulfamic Acid (H₃NSO₃): This strong acid is a popular choice for standardizing bases due to its high purity, stability, and ease of handling.
-
Oxalic Acid Dihydrate (H₂C₂O₄·2H₂O): Another valuable standard for base standardization, but its suitability is dependent on proper storage conditions to avoid dehydration.
These examples highlight the diversity of compounds suitable for different analytical needs. The choice of a primary standard depends heavily on the specific analysis being performed and the analyte’s nature.
Preparing a Primary Standard Solution
The process of preparing a primary standard solution involves meticulous steps to ensure accuracy:
-
Weighing: The primary standard is accurately weighed using an analytical balance. The mass should be recorded to several decimal places.
-
Dissolution: The weighed primary standard is dissolved in a suitable solvent (usually distilled water). Gentle heating might be necessary in some cases, but care must be taken to avoid decomposition.
-
Transfer and Dilution: The solution is quantitatively transferred to a volumetric flask of appropriate volume using a funnel and rinsed thoroughly to ensure all of the standard is transferred. The flask is then filled to the calibration mark with the solvent, ensuring the meniscus is at the mark.
-
Mixing: The solution is thoroughly mixed by inverting the flask several times to achieve a homogeneous concentration.
-
Calculation: The exact molar concentration of the solution is calculated using the mass of the primary standard, its molar mass, and the volume of the solution.
Why Primary Standards are Essential
The use of primary standards is non-negotiable for accurate chemical analysis. Their importance stems from several key reasons:
-
Accurate Concentration Determination: They provide a known and precise concentration, eliminating uncertainties associated with other methods of concentration determination.
-
Reliable Results: The high purity and stability of primary standards ensure that the results of subsequent analyses are reliable and reproducible.
-
Standardization of Solutions: Primary standards are crucial for standardizing solutions of titrants, ensuring the accuracy of volumetric analyses such as titrations.
-
Quality Control: In quality control applications, primary standards help verify the accuracy and precision of analytical instruments and methodologies.
-
Traceability: The use of certified primary standards establishes traceability to internationally recognized standards, increasing confidence in the results.
In short, the use of a primary standard elevates the precision and accuracy of analytical techniques, forming the cornerstone of reliable quantitative chemistry.
Secondary Standards and Their Relationship to Primary Standards
Unlike primary standards, secondary standards are substances whose concentration is determined by comparison with a primary standard. They are often less pure than primary standards and may have limited stability. They are frequently used in situations where using a primary standard might be impractical due to cost or availability. Their use is contingent upon standardization against a primary standard. Their concentrations are indirectly determined. This reliance highlights the fundamental importance of primary standards in the overall reliability of analytical chemistry.
Conclusion
Primary standards are indispensable in analytical chemistry, providing the bedrock for accurate and reliable quantitative analysis. Their characteristics – high purity, stability, known stoichiometry, and ease of handling – ensure that the results obtained using them are dependable. Understanding the criteria for a primary standard, common examples, and the procedure for preparing solutions is crucial for all chemists striving for accurate and reliable experimental results. The meticulous care taken in using and handling primary standards directly translates to the quality and confidence in the overall experimental outcome, underpinning the validity of chemical analysis within research, industry, and education. The role of primary standards extends beyond simple titrations; it forms the bedrock of many quantitative analytical techniques, ensuring the integrity of scientific measurements.
Latest Posts
Latest Posts
-
Number Of Atoms In A Simple Cubic Unit Cell
Apr 02, 2025
-
Inscribed Circle In A Right Triangle
Apr 02, 2025
-
Bacteria And Archaea Are Both Domains Consisting Of Prokaryotic Organisms
Apr 02, 2025
-
What Organelles Do Plants Have That Animals Dont
Apr 02, 2025
-
Select The Five Major Mechanisms Of Antimicrobial Resistance
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Is Primary Standard In Chemistry . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
