What Is Smallest Particle Of An Element
Muz Play
Apr 01, 2025 · 6 min read
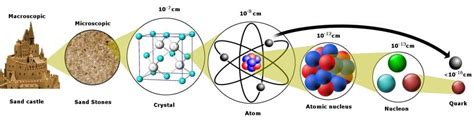
Table of Contents
Delving into the Subatomic World: What is the Smallest Particle of an Element?
The question, "What is the smallest particle of an element?" seems straightforward, but the answer is a journey through the fascinating and often counterintuitive world of quantum mechanics. While the intuitive answer might be the atom, modern physics reveals a much more intricate reality. This article will explore the journey from the atom to the fundamental particles that constitute it, unraveling the mysteries of subatomic structures and shedding light on the complexities of elemental composition.
From Atomos to Atoms: A Historical Perspective
The concept of an indivisible particle of matter has ancient roots. The Greek philosopher Democritus coined the term "atomos," meaning "uncuttable," to describe the fundamental building blocks of the universe. However, this was purely a philosophical concept, lacking experimental evidence. It wasn't until the late 19th and early 20th centuries that scientific investigation confirmed the existence of atoms and revealed their internal structure.
Experiments like J.J. Thomson's cathode ray tube experiment, which discovered the electron, and Ernest Rutherford's gold foil experiment, which unveiled the nucleus, revolutionized our understanding of the atom. These experiments shattered the notion of the atom as a solid, indivisible sphere, unveiling a complex internal structure.
The Atom: Not So Indivisible After All
The atom, while once considered the smallest particle of an element, is actually a composite structure composed of three primary subatomic particles:
1. Protons: Positively Charged Core
Protons reside within the atom's nucleus and carry a positive electrical charge, equal in magnitude to the electron's negative charge. The number of protons in an atom's nucleus defines its atomic number and determines which element it is. For example, an atom with one proton is hydrogen, while an atom with six protons is carbon.
2. Neutrons: Neutral Nuclear Partners
Neutrons, as their name suggests, are electrically neutral particles found alongside protons in the nucleus. They contribute to the atom's mass but not its charge. The number of neutrons in an atom can vary, leading to isotopes of the same element. Isotopes have the same number of protons but different numbers of neutrons.
3. Electrons: Orbiting Negatively Charged Particles
Electrons are negatively charged particles that orbit the nucleus at considerable distances. Their mass is significantly smaller than that of protons and neutrons. The arrangement of electrons in energy levels (or shells) around the nucleus determines the atom's chemical properties and how it interacts with other atoms. The number of electrons typically equals the number of protons in a neutral atom.
Beyond the Atom: Quarks and Leptons – The Fundamental Particles
While protons, neutrons, and electrons were once considered fundamental, further research revealed that protons and neutrons are themselves composed of even smaller particles called quarks. Electrons, on the other hand, belong to a different family of fundamental particles called leptons.
Quarks: The Building Blocks of Protons and Neutrons
There are six types, or "flavors," of quarks: up, down, charm, strange, top, and bottom. Each quark carries a fractional electric charge. Protons are composed of two up quarks and one down quark, while neutrons consist of one up quark and two down quarks. The strong force, mediated by gluons, binds quarks together to form hadrons, which include protons and neutrons.
Leptons: A Different Class of Fundamental Particles
Electrons are the most familiar leptons. Other leptons include muons, tau particles, and their associated neutrinos. Leptons are fundamental particles; they are not composed of smaller constituents. Unlike quarks, they do not experience the strong force.
The Standard Model of Particle Physics
The Standard Model of particle physics is the prevailing theoretical framework that describes the fundamental constituents of matter and their interactions. It incorporates quarks and leptons, along with the force-carrying particles (bosons) that mediate the fundamental forces:
- Electromagnetism: Mediated by photons.
- Weak Nuclear Force: Mediated by W and Z bosons. Responsible for radioactive decay.
- Strong Nuclear Force: Mediated by gluons. Holds quarks together within protons and neutrons.
- Gravity: While not fully integrated into the Standard Model, gravity is a fundamental force acting on all matter.
The Standard Model has been remarkably successful in predicting the behavior of particles and their interactions, but it's not without its limitations. It doesn't include gravity, and some phenomena, like dark matter and dark energy, remain unexplained.
So, What Is the Smallest Particle?
The answer depends on the context. If we're talking about the smallest particle that retains the properties of an element, the answer remains the atom. An atom of hydrogen, for example, is still hydrogen, even though it's composed of smaller particles. Breaking it down further changes its fundamental identity.
However, if we're asking about the smallest fundamental constituents of matter, the answer lies with quarks and leptons. These particles are considered elementary; they are not composed of smaller constituents (as far as we currently know).
Beyond the Standard Model: Open Questions and Future Directions
Despite the success of the Standard Model, there are many unanswered questions that drive ongoing research in particle physics. The search for new particles and a deeper understanding of fundamental forces continues, with experiments like those at the Large Hadron Collider (LHC) pushing the boundaries of our knowledge. Some of the major open questions include:
- The Hierarchy Problem: Why is gravity so much weaker than the other fundamental forces?
- Dark Matter and Dark Energy: What are these mysterious substances that make up the vast majority of the universe's mass-energy content?
- Neutrino Masses and Oscillations: Why do neutrinos have mass, and how do they oscillate between different types?
- Grand Unified Theories (GUTs): Can we unify the electromagnetic, weak, and strong forces into a single framework?
- Supersymmetry (SUSY): Could there be a symmetry between bosons and fermions, predicting a host of new particles?
Conclusion: A Continuous Journey of Discovery
The quest to identify the smallest particle of an element has led us on an extraordinary journey through the subatomic world. While the atom was once considered the ultimate building block, modern physics reveals a much more intricate reality. Quarks and leptons, the fundamental particles described by the Standard Model, currently hold the title of the smallest known constituents of matter. However, the ongoing research in particle physics continues to challenge our understanding, promising further discoveries and a deeper understanding of the universe's fundamental building blocks. The journey of discovery continues, and with each new revelation, our comprehension of the smallest particles and their role in shaping our reality expands. The exploration of the subatomic realm remains a captivating and evolving field, constantly refining our understanding of the fundamental nature of matter and the universe itself.
Latest Posts
Latest Posts
-
Inscribed Circle In A Right Triangle
Apr 02, 2025
-
Bacteria And Archaea Are Both Domains Consisting Of Prokaryotic Organisms
Apr 02, 2025
-
What Organelles Do Plants Have That Animals Dont
Apr 02, 2025
-
Select The Five Major Mechanisms Of Antimicrobial Resistance
Apr 02, 2025
-
What Are The Three Characteristics Of All Metals
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Is Smallest Particle Of An Element . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
