What Is The Boiling Point And Freezing Point Of Water
Muz Play
Mar 30, 2025 · 6 min read
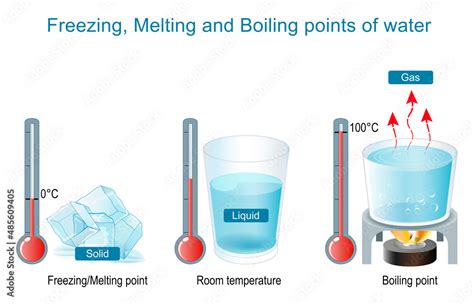
Table of Contents
What is the Boiling Point and Freezing Point of Water? A Deep Dive
Water, the elixir of life, is a substance so fundamental to our existence that we often take its properties for granted. Yet, understanding the seemingly simple characteristics of water, such as its boiling and freezing points, reveals a complex interplay of molecular forces and physical phenomena with far-reaching implications for our planet and beyond. This comprehensive article delves into the precise definitions, the underlying science, and the factors that can influence the boiling and freezing points of water.
Defining Boiling and Freezing Points
Before we delve into the specifics, let's clarify the definitions of boiling and freezing points.
Boiling Point
The boiling point of a liquid is the temperature at which its vapor pressure equals the external pressure surrounding the liquid. At this point, the liquid transforms into a gas (vapor) throughout its volume, not just at the surface (like evaporation). For water, under standard atmospheric pressure (1 atmosphere or 101.325 kPa), the boiling point is precisely 100 degrees Celsius (212 degrees Fahrenheit).
Freezing Point
The freezing point, on the other hand, is the temperature at which a liquid transforms into a solid. For water, this occurs at 0 degrees Celsius (32 degrees Fahrenheit) under standard atmospheric pressure. This transition involves the formation of a crystalline structure – ice – where water molecules arrange themselves in a specific, ordered pattern.
The Science Behind the Points: Intermolecular Forces
The boiling and freezing points of water aren't arbitrary numbers; they are direct consequences of the intermolecular forces between water molecules. Water molecules (H₂O) are polar, meaning they have a slightly positive end (the hydrogen atoms) and a slightly negative end (the oxygen atom). This polarity leads to several key interactions:
Hydrogen Bonding: The Key Player
The most significant intermolecular force in water is hydrogen bonding. This relatively strong type of dipole-dipole attraction occurs between the slightly positive hydrogen atom of one water molecule and the slightly negative oxygen atom of another. These hydrogen bonds create a cohesive network holding water molecules together.
Impact on Boiling Point: To boil water, you need to overcome these hydrogen bonds, supplying enough energy to separate the molecules and allow them to escape into the gaseous phase. The relatively strong hydrogen bonds explain why water has a higher boiling point than many other similarly sized molecules. For instance, methane (CH₄), a molecule of similar size, has a boiling point of -161.5 °C because it lacks hydrogen bonding.
Impact on Freezing Point: During freezing, the hydrogen bonds become even more crucial. They dictate the formation of the ice crystal lattice, a highly ordered hexagonal structure. The energy required to break these bonds during melting contributes to the relatively high melting point of water compared to many other substances.
Other Intermolecular Forces
While hydrogen bonding dominates, other weaker forces, such as van der Waals forces, also play a role. These forces are present between all molecules, but they are significantly weaker than hydrogen bonds in water.
Factors Affecting Boiling and Freezing Points
While 100°C and 0°C are the standard boiling and freezing points of water, these values are not immutable. Several factors can influence them:
Pressure
As mentioned earlier, the boiling point of water is directly related to the external pressure. At higher altitudes, where atmospheric pressure is lower, water boils at a lower temperature. Conversely, at higher pressures, the boiling point increases. This is why pressure cookers work; the increased pressure allows water to reach higher temperatures, cooking food faster.
The freezing point is also affected by pressure, but to a much lesser extent than the boiling point. However, under very high pressures, the freezing point of water can actually decrease slightly.
Impurities
The presence of dissolved impurities in water can also affect its boiling and freezing points. Generally, adding solutes (like salt) raises the boiling point (boiling point elevation) and lowers the freezing point (freezing point depression). This is why salting roads in winter helps to melt ice—the salt lowers the freezing point of water, preventing ice formation at typical winter temperatures. This phenomenon is explained by colligative properties, which depend on the concentration of solute particles, not their identity.
Isotopes
Even the isotopic composition of water can slightly alter its properties. "Heavy water," containing deuterium (²H) instead of ordinary hydrogen (¹H), has a slightly higher boiling point (101.4 °C) and freezing point (3.8 °C) than regular water.
The Anomalous Properties of Water
Water exhibits several properties that are considered anomalous, meaning they are unusual compared to other substances. These anomalies are directly linked to its strong hydrogen bonding and its unique molecular structure.
Density Anomaly
The most striking anomaly is the density of water. Most substances become denser as they freeze, but ice is less dense than liquid water. This is because the hydrogen bonds in ice create a relatively open, crystalline structure with more space between molecules. This lower density is crucial for aquatic life, allowing ice to float on the surface, insulating the water beneath and preventing it from freezing completely.
High Specific Heat Capacity
Water also possesses an exceptionally high specific heat capacity, meaning it can absorb a large amount of heat with a relatively small temperature change. This property is vital in regulating Earth's climate, moderating temperature fluctuations, and supporting diverse ecosystems.
High Heat of Vaporization
Water also has a high heat of vaporization, meaning it requires a significant amount of energy to transform from liquid to vapor. This property plays a crucial role in cooling processes like sweating in humans and transpiration in plants.
Applications and Significance
Understanding the boiling and freezing points of water is essential in numerous fields:
- Cooking: Boiling and freezing are fundamental processes in food preparation and preservation.
- Chemistry: These points are crucial in determining the purity of water and in various chemical reactions and experiments.
- Engineering: Engineers utilize these properties in designing cooling systems, heat exchangers, and other applications.
- Meteorology: The phase transitions of water are central to weather patterns, precipitation, and climate change.
- Biology: The unique properties of water are fundamental to life itself, influencing biological processes at every level.
Conclusion: Beyond the Numbers
The boiling and freezing points of water, while seemingly simple numbers (100°C and 0°C under standard conditions), encapsulate a rich tapestry of scientific principles. The strong hydrogen bonds between water molecules, alongside other intermolecular forces, drive these properties and contribute to water’s unique and anomalous behavior. These properties are not just academic curiosities; they are fundamental to the functioning of our planet's ecosystems, the development of technology, and the very existence of life as we know it. Further research and understanding of these seemingly simple points continue to unveil the profound complexity and significance of water in our world.
Latest Posts
Latest Posts
-
How Do You Calculate Stream Gradient
Apr 01, 2025
-
What Happens To A Cell In A Hypertonic Solution
Apr 01, 2025
-
Titration Of Fruit Juice Lab Answers
Apr 01, 2025
-
Electric Field Lines About A Point Charge Extend
Apr 01, 2025
-
Does Ionization Energy Increase Across A Period
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Is The Boiling Point And Freezing Point Of Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
