What Happens To A Cell In A Hypertonic Solution
Muz Play
Apr 01, 2025 · 6 min read
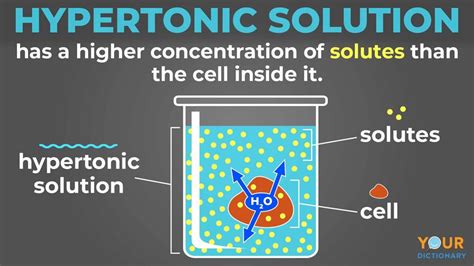
Table of Contents
What Happens to a Cell in a Hypertonic Solution? A Comprehensive Guide
Understanding cellular responses to different environments is fundamental to biology. One crucial concept is the impact of tonicity, specifically hypertonic solutions, on cells. This comprehensive guide delves deep into the processes that occur when a cell is placed in a hypertonic solution, exploring the underlying mechanisms, consequences, and implications across various cell types.
Defining Hypertonic Solutions and Osmosis
Before exploring the cellular effects, let's establish the foundation: what is a hypertonic solution? A hypertonic solution is one in which the concentration of solutes (dissolved substances) is higher outside the cell than inside. This concentration difference drives a process called osmosis.
Osmosis is the passive movement of water across a selectively permeable membrane from a region of high water concentration (low solute concentration) to a region of low water concentration (high solute concentration). The selectively permeable membrane, in this case, is the cell membrane, which allows water to pass through but restricts the movement of many solutes.
The Cellular Response: Water Loss and Plasmolysis
When a cell is immersed in a hypertonic solution, water begins to flow out of the cell. This is because the water concentration is higher inside the cell than outside. This outward movement of water leads to several significant changes within the cell:
1. Cell Shrinkage:
The most immediate effect is a reduction in cell volume. The cell shrinks as water is lost, a process often referred to as plasmolysis. The degree of shrinkage depends on several factors including the concentration difference between the internal and external environments, the cell wall's rigidity (if present), and the cell's initial water content.
2. Membrane Detachment (in Plant Cells):
Plant cells possess a rigid cell wall surrounding the plasma membrane. During plasmolysis in plant cells, the plasma membrane pulls away from the cell wall, a process known as plasmolysis. This detachment is visible under a microscope. The space created between the membrane and cell wall is filled with the hypertonic solution.
3. Cytoplasmic Changes:
As the cell shrinks, the cytoplasm, the jelly-like substance within the cell, also shrinks and becomes more concentrated. This increased concentration can affect various cellular processes, potentially leading to changes in metabolic activity. Organelles within the cytoplasm may also be affected, potentially leading to a disruption in their function.
4. Altered Cellular Function:
The loss of water and subsequent changes within the cell significantly impact its function. Enzyme activity can be affected by the altered concentration of solutes and the changes in the internal environment. Transport processes across the cell membrane may be disrupted due to the changes in membrane structure and fluidity. Protein synthesis and other essential metabolic pathways can be impaired.
Varied Responses Across Cell Types
The response of cells to hypertonic solutions isn't uniform across all cell types. The presence or absence of a cell wall, the cell's initial water content, and the nature of the solute in the hypertonic solution all influence the degree and nature of the response.
Plant Cells vs. Animal Cells:
-
Plant Cells: The rigid cell wall provides structural support and limits the extent of shrinkage. Although plasmolysis occurs, the cell wall prevents complete collapse. When the cell is reintroduced to a hypotonic or isotonic solution, the cell can often recover its turgor pressure.
-
Animal Cells: Animal cells lack a rigid cell wall, making them much more susceptible to the effects of hypertonic solutions. The cell can shrink dramatically, potentially leading to cell death if the water loss is severe enough. The disruption of cellular function and structural integrity can result in irreversible damage.
Bacterial Cells:
Bacterial cells, like plant cells, have a cell wall, but the wall composition differs. The response to a hypertonic solution will vary depending on the bacterial species and the specific composition of the cell wall. Some bacteria may exhibit plasmolysis, while others might develop mechanisms to counteract the water loss.
Other Eukaryotic Cells:
Fungal cells and protist cells, both eukaryotic organisms, also demonstrate varied responses to hypertonic stress depending on their specific characteristics and adaptations.
Adaptations and Mechanisms of Tolerance
Some organisms and cells have evolved mechanisms to tolerate hypertonic environments:
-
Osmoregulation: Many organisms possess sophisticated osmoregulatory mechanisms to maintain a stable internal water balance, even in hypertonic conditions. This often involves actively transporting ions and other solutes to adjust the osmotic pressure.
-
Compatible Solutes: Some cells synthesize and accumulate compatible solutes (e.g., proline, glycine betaine) within the cytoplasm. These solutes do not interfere with cellular functions but help to maintain osmotic balance and prevent water loss.
-
Aquaporins: Aquaporins are water channels that facilitate the rapid movement of water across the cell membrane. In some cases, the regulation of aquaporin activity can play a role in responding to hypertonic stress.
Implications and Applications
The effects of hypertonic solutions on cells have various implications in different fields:
Medicine:
Understanding the effects of hypertonic solutions is crucial in various medical applications. For example, hypertonic saline solutions are sometimes used in treating certain medical conditions. The osmotic effect can help to reduce swelling or draw fluid out of tissues.
Food Preservation:
Hypertonic solutions, such as concentrated salt or sugar solutions, are often used in food preservation. The high solute concentration prevents microbial growth by drawing water out of microbial cells, inhibiting their metabolic activity.
Agriculture:
Understanding the impact of salinity (high salt concentration) on plant cells is vital in agriculture, as salinity stress can significantly affect crop yields. Scientists are working to develop salt-tolerant crop varieties that can withstand hypertonic conditions in saline soils.
Conclusion: A Dynamic Cellular Response
The response of a cell to a hypertonic solution is a dynamic process driven by osmosis. Water loss, plasmolysis, and changes in cellular function are common consequences. The severity of these effects depends on factors like cell type, the magnitude of the osmotic gradient, and the presence of adaptations. Understanding these processes is fundamental to comprehending various biological phenomena and has significant implications across numerous fields including medicine, agriculture, and food science. Further research continually expands our knowledge of the complex cellular mechanisms involved in responding to hypertonic stress and opens avenues for manipulating these processes to various beneficial ends.
Further exploration could delve into specific examples of hypertonic solutions used in medicine (e.g., mannitol, hypertonic saline), detailed explanations of osmoregulation in different organisms (e.g., freshwater fish vs. saltwater fish), and the molecular mechanisms behind the synthesis and accumulation of compatible solutes. The impact of hypertonic stress on cell signaling pathways and gene expression is also a rich area for further research and understanding.
Latest Posts
Latest Posts
-
Draw A Structural Formula For 3 Bromo 4 Chloro 1 1 Dimethylcyclohexane
Apr 02, 2025
-
What Is A Primary Standard Chemistry
Apr 02, 2025
-
How Do You Convert From Atoms To Grams
Apr 02, 2025
-
How Do Positive Ions And Negative Ions Form
Apr 02, 2025
-
Density Of Water At Different Temperatures Chart
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Happens To A Cell In A Hypertonic Solution . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
