What Is The Main Difference Between Protons And Neutrons
Muz Play
Mar 30, 2025 · 6 min read
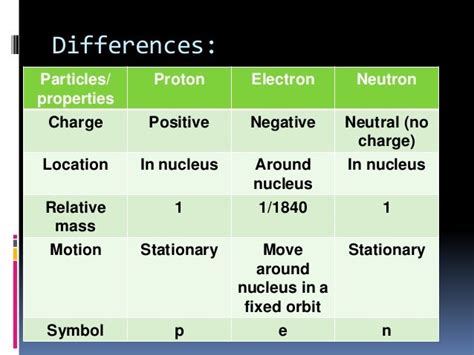
Table of Contents
What's the Main Difference Between Protons and Neutrons? A Deep Dive into Subatomic Particles
The atom, once considered the indivisible building block of matter, has revealed a fascinatingly complex inner world. At its core lie protons and neutrons, collectively known as nucleons, residing within the atom's nucleus. While they share a similar mass, their fundamental differences drive the behavior of atoms and, consequently, the properties of matter. This article delves into the key distinctions between protons and neutrons, exploring their composition, charge, interactions, and roles in the structure of matter.
The Fundamental Difference: Electric Charge
The most significant difference between protons and neutrons lies in their electric charge. Protons carry a positive charge, while neutrons are electrically neutral. This seemingly simple distinction has profound consequences for the atom's structure and its interactions with other atoms and particles. The positive charge of protons is responsible for the electrostatic attraction that binds electrons, negatively charged particles, to the nucleus. This attractive force is the foundation of the atom's stability and its chemical properties. Neutrons, lacking any charge, do not participate directly in these electrostatic interactions.
Implications of Charge Difference:
- Atomic Number: The number of protons in an atom's nucleus defines its atomic number, a unique identifier for each element on the periodic table. For instance, hydrogen has one proton (atomic number 1), helium has two (atomic number 2), and so on. This number dictates the element's chemical behavior.
- Isotopes: The number of neutrons in an atom's nucleus can vary, even for atoms of the same element. These variations are called isotopes. For example, carbon-12 has six protons and six neutrons, while carbon-14 has six protons and eight neutrons. Isotopes exhibit similar chemical properties due to the identical number of protons but may differ in their physical properties and nuclear stability.
- Ionic Bonds: The positive charge of protons plays a crucial role in the formation of ionic bonds. When atoms lose or gain electrons to achieve a stable electron configuration, they become ions, carrying a net positive or negative charge. The electrostatic attraction between oppositely charged ions forms ionic bonds, a fundamental type of chemical bond.
Composition and Internal Structure: Quarks
Both protons and neutrons are not fundamental particles; they are composed of even smaller constituents called quarks. This subatomic level unveils another layer of complexity in understanding the differences between these nucleons.
Proton Composition:
A proton comprises two up quarks and one down quark. Each up quark carries a charge of +2/3, while the down quark carries a charge of -1/3. The combined charge of these three quarks results in the proton's overall positive charge of +1.
Neutron Composition:
A neutron consists of one up quark and two down quarks. With the same individual quark charges, the combined charge of a neutron is zero, resulting in its electrical neutrality.
Implications of Quark Composition:
- Strong Nuclear Force: The quarks within protons and neutrons are bound together by the strong nuclear force, one of the four fundamental forces in nature. This force is significantly stronger than the electromagnetic force and is responsible for holding the nucleus together, despite the repulsive electrostatic forces between the positively charged protons.
- Hadrons: Protons and neutrons belong to a class of particles called hadrons, which are composite particles made up of quarks.
- Quantum Chromodynamics (QCD): The theory governing the interaction of quarks and the strong nuclear force is quantum chromodynamics (QCD). QCD is a complex theory that explains the properties of hadrons and their interactions.
Mass and Size: Subtle Differences
While protons and neutrons have very similar masses, there are slight differences.
- Proton Mass: Approximately 1.6726 × 10^-27 kg.
- Neutron Mass: Slightly larger than a proton, approximately 1.6749 × 10^-27 kg.
This small mass difference has implications for nuclear stability and radioactive decay. The size of protons and neutrons is also similar, on the order of 1 femtometer (10^-15 meters), although defining a precise size is challenging due to the complex nature of their internal structure.
Nuclear Stability and Radioactive Decay: The Neutron's Role
The relative number of protons and neutrons in an atom's nucleus plays a vital role in determining its stability. For lighter elements, a roughly equal number of protons and neutrons generally leads to stable isotopes. However, as the atomic number increases, a higher proportion of neutrons is required for nuclear stability. This is because the strong nuclear force, which holds the nucleus together, has a shorter range than the electromagnetic force repelling the protons. Extra neutrons help to overcome the proton-proton repulsion.
Neutrons also play a critical role in radioactive decay, a process by which unstable atomic nuclei lose energy by emitting radiation. Several types of radioactive decay involve neutrons, including beta decay, where a neutron transforms into a proton, an electron, and an antineutrino. This conversion alters the atomic number and thus the element's identity.
Summary Table: Proton vs. Neutron
| Feature | Proton | Neutron |
|---|---|---|
| Electric Charge | +1 | 0 |
| Mass | 1.6726 × 10^-27 kg | 1.6749 × 10^-27 kg |
| Quark Composition | 2 up quarks, 1 down quark | 1 up quark, 2 down quarks |
| Role in Atom | Determines atomic number, contributes to nuclear stability | Contributes to nuclear stability, involved in some radioactive decays |
| Symbol | p⁺ or p | n⁰ or n |
Beyond the Basics: Further Exploration
The differences between protons and neutrons extend beyond their fundamental properties. Research into these subatomic particles continues to uncover new insights into the fundamental forces governing the universe. Areas of ongoing investigation include:
- Neutron Stars: These incredibly dense celestial objects are composed primarily of neutrons, formed from the remnants of massive stars after supernova explosions. Studying neutron stars provides valuable information about the behavior of matter under extreme conditions of density and pressure.
- The Strong Nuclear Force: Understanding the complexities of the strong force remains a significant challenge. Research in particle physics aims to unravel the intricate interactions between quarks and gluons, the particles mediating the strong force.
- Exotic Hadrons: Beyond the familiar protons and neutrons, physicists have discovered exotic hadrons, particles containing more than three quarks or combinations of quarks and antiquarks. These particles provide valuable clues to the fundamental building blocks of matter.
In conclusion, while protons and neutrons share similarities in mass and size, their crucial difference lies in their electric charge. This seemingly simple difference underpins the fundamental structure of matter, influences chemical bonding, determines atomic identity, and plays a central role in nuclear stability and radioactive decay. Their internal structure, composed of quarks, further illuminates the intricate interplay of fundamental forces that govern the universe at its most basic level. The ongoing study of these nucleons continues to reveal new insights into the nature of matter and the forces that shape it.
Latest Posts
Latest Posts
-
Which Will Increase The Rate Of A Chemical Reaction
Apr 01, 2025
-
How Are Electrons Arranged Around The Nucleus Of An Atom
Apr 01, 2025
-
Does Plasma Have A Difinite Volume
Apr 01, 2025
-
Stack Of Membranes That Packages Chemicals
Apr 01, 2025
-
Arrange The Compounds By Their Solubility In Water
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Is The Main Difference Between Protons And Neutrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
