What Is The Rule For Adding Decimals Ap Chemistry
Muz Play
Mar 16, 2025 · 5 min read
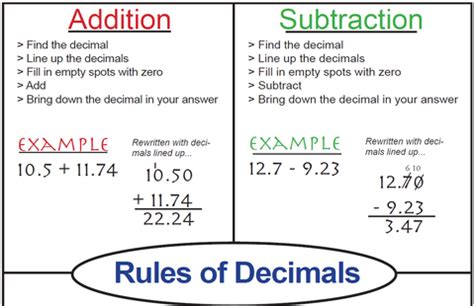
Table of Contents
What Are the Rules for Adding Decimals in AP Chemistry? A Comprehensive Guide
Adding decimals might seem like a simple task, but in the rigorous world of AP Chemistry, precision is paramount. A seemingly minor error in decimal addition can lead to significantly inaccurate calculations and flawed conclusions. This comprehensive guide delves into the rules and nuances of adding decimals in AP Chemistry, equipping you with the knowledge and skills to confidently tackle even the most complex calculations.
Understanding Significant Figures and Their Role in Decimal Addition
Before diving into the mechanics of decimal addition, it's crucial to grasp the concept of significant figures (sig figs). Significant figures represent the digits in a number that carry meaning contributing to its precision. They're fundamental in AP Chemistry because they dictate the level of accuracy we can report in our calculations and experimental results. Incorrect handling of sig figs can lead to inaccurate representation of data.
Identifying Significant Figures
Here's a quick refresher on identifying significant figures:
- Non-zero digits: All non-zero digits are always significant. (e.g., in 25.3, all three digits are significant)
- Zeros:
- Leading zeros: Zeros placed before non-zero digits are not significant (e.g., in 0.0025, only 2 and 5 are significant)
- Trailing zeros: Trailing zeros in a number without a decimal point are not significant (e.g., in 100, only 1 is significant). However, in a number with a decimal point, trailing zeros are significant (e.g., in 100.0, all three zeros are significant).
- Captive zeros: Zeros between non-zero digits are significant (e.g., in 1005, all four digits are significant).
The Rule for Adding Decimals with Sig Figs
The rule for adding decimals involving significant figures is straightforward: The final answer should have the same number of decimal places as the measurement with the fewest decimal places. This ensures that the result doesn't imply a greater precision than the original data warrants.
Example 1:
Add the following measurements:
- 25.3 g
- 10.05 g
- 0.225 g
Solution:
-
Perform the addition: 25.3 + 10.05 + 0.225 = 35.575 g
-
Identify the measurement with the fewest decimal places: 25.3 g (one decimal place).
-
Round the result to one decimal place: 35.6 g
Therefore, the correct answer is 35.6 g. Note that even though our initial calculation yielded 35.575 g, we rounded down to 35.6 g to maintain consistency with the significant figures of the least precise measurement.
Dealing with Scientific Notation in Decimal Addition
Scientific notation is often used in AP Chemistry to represent very large or very small numbers concisely. When adding numbers in scientific notation, it's crucial to follow these steps:
-
Convert to the same power of 10: Before adding numbers in scientific notation, ensure that they all have the same power of 10. You may need to adjust the coefficient and the exponent accordingly.
-
Add the coefficients: Once the powers of 10 are identical, add the coefficients (the numbers before the power of 10).
-
Maintain the power of 10: The power of 10 remains unchanged during the addition.
-
Apply significant figure rules: After adding the coefficients, apply the significant figure rules to the final result, as discussed earlier.
Example 2:
Add the following measurements:
- 2.5 x 10² mL
- 1.05 x 10¹ mL
Solution:
-
Convert to the same power of 10: 1.05 x 10¹ mL = 0.105 x 10² mL
-
Add the coefficients: 2.5 + 0.105 = 2.605
-
Maintain the power of 10: 2.605 x 10² mL
-
Apply significant figures: The least precise measurement (2.5 x 10²) has two significant figures, so the final answer should also have two significant figures. Therefore, we round 2.605 to 2.6.
The final answer is 2.6 x 10² mL.
Common Mistakes to Avoid When Adding Decimals in AP Chemistry
Several common mistakes can lead to inaccuracies in decimal addition in AP Chemistry. These include:
-
Ignoring significant figures: Failing to consider significant figures is a widespread error that can significantly affect the accuracy of your results. Always double-check your work against the significant figure rules.
-
Incorrect rounding: Rounding prematurely or incorrectly can lead to cumulative errors, especially in multi-step calculations. Only round at the very end of your calculation to minimize error propagation.
-
Misalignment of decimal points: Carefully align the decimal points when adding decimals vertically. A simple misalignment can lead to significant errors in your answer.
-
Forgetting unit consistency: Make sure that all your measurements are in the same units before adding them. You can't add grams to kilograms without converting them to the same unit first.
-
Using an incorrect number of decimal places: Remember that when adding decimal numbers, you should round your answer to the same number of decimal places as the measurement with the fewest decimal places, not necessarily the number of decimal places in your original numbers.
Advanced Scenarios: Handling Logarithms and Exponential Functions
While the basic rules for adding decimals remain the same, certain AP Chemistry concepts, such as logarithmic and exponential functions, require additional considerations. In these cases, it's crucial to adhere to the order of operations (PEMDAS/BODMAS) and to maintain consistency in your units and significant figures throughout the calculation. Remember, the rules of significant figures are still crucial here: The final answer should reflect the precision of the least precise input value.
Conclusion: Mastering Decimal Addition for AP Chemistry Success
Mastering decimal addition and understanding significant figures are indispensable skills for success in AP Chemistry. By diligently applying the rules outlined in this guide and avoiding common pitfalls, you can ensure the accuracy and precision of your calculations, leading to a stronger understanding of chemical principles and a higher score on your AP exam. Practice is key – the more you work with these concepts, the more confident and proficient you'll become in handling decimal addition in even the most complex chemical calculations. Remember that attention to detail and a thorough understanding of significant figures are your greatest allies in achieving accuracy in your AP Chemistry work.
Latest Posts
Latest Posts
-
Can A Buffer Be Made With A Strong Acid
Mar 17, 2025
-
Gas Laws Practice Problems With Answers
Mar 17, 2025
-
Are Strong Bases Good Leaving Groups
Mar 17, 2025
-
Which Polymer Is Composed Of Amino Acids
Mar 17, 2025
-
According To Dalton Atoms Of Different Elements Will Be
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Is The Rule For Adding Decimals Ap Chemistry . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
