When An Atom Gains An Electron It Becomes
Muz Play
Mar 21, 2025 · 6 min read
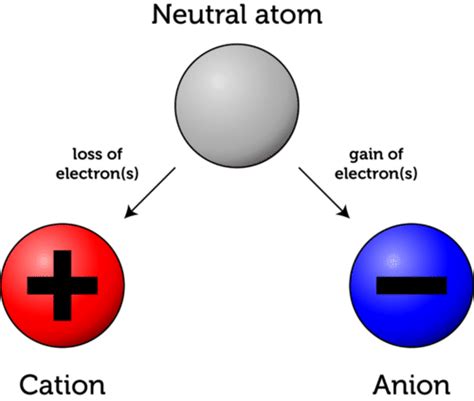
Table of Contents
When an Atom Gains an Electron: Delving into the World of Ions and Chemical Reactions
When an atom gains an electron, it undergoes a fundamental transformation, shifting from a neutral state to a negatively charged entity known as an anion. This seemingly simple process has profound implications across numerous scientific disciplines, influencing the behavior of matter at both the microscopic and macroscopic levels. Understanding this transformation requires exploring the fundamental concepts of atomic structure, electron configuration, and the driving forces behind electron transfer.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before delving into the consequences of electron gain, let's revisit the basic building blocks of an atom. An atom is composed of three primary subatomic particles:
- Protons: Positively charged particles residing in the atom's nucleus. The number of protons defines the element's atomic number and determines its chemical identity.
- Neutrons: Neutrally charged particles also located within the nucleus. They contribute to the atom's mass but not its charge.
- Electrons: Negatively charged particles orbiting the nucleus in specific energy levels or shells. These electrons are crucial for chemical bonding and reactivity.
In a neutral atom, the number of protons equals the number of electrons, resulting in a net charge of zero. This balance is what governs the atom's stability and its interaction with other atoms.
The Significance of Electron Shells and Valence Electrons
Electrons are arranged in distinct energy levels or shells surrounding the nucleus. The outermost shell, known as the valence shell, contains the valence electrons. These electrons play a critical role in chemical reactions, as they are the most loosely bound to the nucleus and are most readily involved in interactions with other atoms.
The number of valence electrons dictates an atom's reactivity and its tendency to gain, lose, or share electrons to achieve a stable electron configuration. Atoms strive to achieve a full valence shell, often resembling the electron configuration of the nearest noble gas (Group 18 elements). This stable configuration is often referred to as the octet rule, although there are exceptions, particularly with elements in periods beyond the third.
The Process of Electron Gain: Forming Anions
When an atom gains an electron, it acquires a negative charge because the number of electrons now exceeds the number of protons. This negatively charged atom is called an anion. The process of anion formation is often driven by the atom's desire to achieve a more stable electron configuration, typically by filling its valence shell.
For instance, consider a chlorine atom (Cl). Chlorine has seven valence electrons. By gaining one electron, it achieves a full valence shell of eight electrons, matching the stable configuration of the noble gas argon (Ar). This transformation results in the formation of a chloride anion (Cl⁻).
The notation for anions includes a superscript minus sign (-) indicating the negative charge, with the number of minus signs reflecting the number of electrons gained.
Factors Influencing Electron Gain
Several factors influence an atom's propensity to gain electrons:
- Electronegativity: Electronegativity measures an atom's ability to attract electrons towards itself in a chemical bond. Atoms with high electronegativity, such as those in Group 17 (halogens), have a strong tendency to gain electrons.
- Ionization Energy: Ionization energy is the energy required to remove an electron from an atom. Atoms with low ionization energies tend to lose electrons more readily, while atoms with high ionization energies are less likely to do so. Conversely, atoms with high electron affinity (the energy released when an electron is added to a neutral atom) will more readily gain electrons.
- Atomic Size: Larger atoms tend to have lower ionization energies and higher electron affinities, making them more likely to gain electrons. The increased distance between the nucleus and the outermost electrons weakens the electrostatic attraction, making it easier to add an electron.
Consequences of Anion Formation: Chemical and Physical Properties
The transformation of a neutral atom into an anion significantly alters its chemical and physical properties:
- Chemical Reactivity: Anions are generally less reactive than their corresponding neutral atoms. The gained electron fills the valence shell, making the anion less likely to participate in further electron transfer reactions. However, anions can still engage in ionic bonding with cations (positively charged ions).
- Ionic Bonding: Anions are fundamental to ionic bonding, a type of chemical bond formed between oppositely charged ions. The electrostatic attraction between cations and anions holds the ions together in a crystal lattice structure. Examples of ionic compounds include sodium chloride (NaCl), where sodium forms a cation (Na⁺) and chlorine forms an anion (Cl⁻).
- Physical Properties: The physical properties of an anion, such as its size, melting point, and boiling point, differ from those of its corresponding neutral atom. Anions are generally larger than their neutral counterparts due to the added electron, leading to increased electron-electron repulsion and a larger ionic radius. The melting and boiling points of ionic compounds are typically high due to the strong electrostatic attraction between the ions.
- Solubility: The solubility of ionic compounds depends on the polarity of the solvent and the strength of the ionic bonds. Polar solvents, such as water, effectively dissolve many ionic compounds by surrounding the ions and weakening the electrostatic interactions.
Examples of Anion Formation: Exploring Specific Elements
Let's examine some specific examples of anion formation:
- Oxygen (O): Oxygen has six valence electrons. By gaining two electrons, it forms the oxide anion (O²⁻), achieving a stable octet.
- Sulfur (S): Sulfur, like oxygen, has six valence electrons. It also forms a stable anion, the sulfide anion (S²⁻), by gaining two electrons.
- Nitrogen (N): Nitrogen has five valence electrons. It can gain three electrons to form the nitride anion (N³⁻).
- Fluorine (F): Fluorine, a halogen, has seven valence electrons and readily gains one electron to form the fluoride anion (F⁻).
These examples highlight the common theme of atoms gaining electrons to achieve a stable electron configuration, thereby fulfilling the octet rule in many instances.
Anions in Biological Systems and Everyday Life
Anions are not merely abstract concepts; they play vital roles in numerous biological systems and everyday life:
- Biological Systems: Many biological molecules contain anions, including phosphate (PO₄³⁻) ions, crucial for energy transfer in cells (ATP), and chloride (Cl⁻) ions, essential for maintaining fluid balance in the body.
- Everyday Life: Many compounds encountered in everyday life contain anions. Sodium chloride (table salt) is a ubiquitous example. Other examples include calcium carbonate (CaCO₃), found in limestone and seashells, and bicarbonate ions (HCO₃⁻), which act as buffers in blood, maintaining a stable pH.
Conclusion: The Broader Significance of Electron Gain
The seemingly simple process of an atom gaining an electron has far-reaching consequences, shaping the chemical and physical properties of matter and influencing the behavior of substances at both the atomic and macroscopic levels. Understanding the principles of atomic structure, electron configuration, and the driving forces behind electron transfer is crucial to comprehending a wide range of phenomena in chemistry, biology, materials science, and numerous other fields. From the formation of ionic compounds to the function of biological molecules, the gain of an electron is a fundamental process with profound implications for the world around us. Further exploration of this topic involves delving into redox reactions, electrochemistry, and advanced concepts in quantum mechanics, each revealing further nuances and complexities of this fundamental aspect of atomic behavior.
Latest Posts
Latest Posts
-
How Does Atomic Radius Increase Across The Periodic Table
Mar 22, 2025
-
Label The Microscopic Anatomy Of Spongy Bone
Mar 22, 2025
-
Level Of Analysis In International Relations
Mar 22, 2025
-
Label The Structures Of The Vertebra
Mar 22, 2025
-
How Many Types Of Speech Are There
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about When An Atom Gains An Electron It Becomes . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
