Which Electrons Are Involved In Chemical Bonding
Muz Play
Apr 01, 2025 · 6 min read
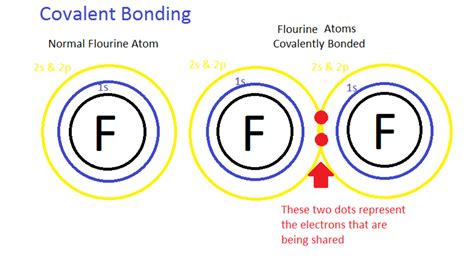
Table of Contents
Which Electrons are Involved in Chemical Bonding?
Chemical bonding, the force that holds atoms together to form molecules and compounds, is a fundamental concept in chemistry. Understanding which electrons participate in this process is crucial to grasping the nature of chemical interactions and predicting the properties of substances. This article delves deep into the intricacies of chemical bonding, focusing specifically on the electrons responsible for these interactions.
Valence Electrons: The Key Players
The primary electrons involved in chemical bonding are valence electrons. These are the electrons located in the outermost shell, or energy level, of an atom. They are farthest from the atom's nucleus and experience the weakest attraction to the positively charged protons within the nucleus. This weaker attraction makes them more readily available to participate in interactions with other atoms.
Understanding Electron Shells and Subshells
Before diving deeper, let's briefly review the organization of electrons within an atom. Electrons occupy specific energy levels, often depicted as shells. Each shell has a maximum capacity for electrons, determined by the formula 2n², where 'n' is the shell number (n=1 for the first shell, n=2 for the second, and so on). Furthermore, shells are subdivided into subshells (s, p, d, and f), each capable of holding a specific number of electrons.
- s subshell: Holds a maximum of 2 electrons.
- p subshell: Holds a maximum of 6 electrons.
- d subshell: Holds a maximum of 10 electrons.
- f subshell: Holds a maximum of 14 electrons.
The arrangement of electrons in these shells and subshells is described by the electron configuration of the atom. This configuration dictates an atom's chemical behavior and its propensity to form bonds.
The Significance of Valence Electrons in Bonding
Valence electrons are crucial because they determine an atom's valency, or the number of bonds it can form. Atoms tend to achieve a stable electron configuration, often resembling that of a noble gas (Group 18 elements). This stable configuration, typically characterized by a full outermost shell (octet rule for most elements), is achieved through the gain, loss, or sharing of valence electrons.
Types of Chemical Bonds and Electron Involvement
Several types of chemical bonds exist, each involving valence electrons in unique ways:
1. Ionic Bonds: Transfer of Electrons
Ionic bonds form through the complete transfer of one or more valence electrons from one atom to another. This transfer results in the formation of ions: positively charged cations (atoms that have lost electrons) and negatively charged anions (atoms that have gained electrons). The electrostatic attraction between these oppositely charged ions constitutes the ionic bond.
Example: Consider the formation of sodium chloride (NaCl), common table salt. Sodium (Na) has one valence electron, while chlorine (Cl) has seven. Sodium readily loses its valence electron to achieve a stable octet, becoming a Na⁺ cation. Chlorine gains this electron to complete its octet, becoming a Cl⁻ anion. The electrostatic attraction between Na⁺ and Cl⁻ forms the ionic bond in NaCl.
2. Covalent Bonds: Sharing of Electrons
Covalent bonds involve the sharing of one or more pairs of valence electrons between two atoms. This sharing allows both atoms to achieve a more stable electron configuration. The shared electrons are considered to belong to both atoms simultaneously.
Types of Covalent Bonds:
- Single covalent bonds: Involve the sharing of one pair of electrons. For example, in a hydrogen molecule (H₂), each hydrogen atom shares its single valence electron with the other, forming a single covalent bond.
- Double covalent bonds: Involve the sharing of two pairs of electrons. For example, in an oxygen molecule (O₂), each oxygen atom shares two pairs of electrons with the other, resulting in a double covalent bond.
- Triple covalent bonds: Involve the sharing of three pairs of electrons. For example, in a nitrogen molecule (N₂), each nitrogen atom shares three pairs of electrons with the other, forming a triple covalent bond.
Polar Covalent Bonds: When electrons are shared unequally between two atoms, due to differences in electronegativity (the ability of an atom to attract electrons in a bond), a polar covalent bond is formed. This results in a slightly positive end and a slightly negative end within the molecule. Water (H₂O) is a classic example of a molecule with polar covalent bonds.
3. Metallic Bonds: Delocalized Electrons
Metallic bonds occur in metals. In this type of bonding, valence electrons are not localized to individual atoms but are instead delocalized, forming a "sea" of electrons that are free to move throughout the metal lattice. This delocalized electron sea holds the positively charged metal ions together. The mobility of these electrons accounts for the characteristic properties of metals, such as high electrical and thermal conductivity and malleability.
Factors Influencing Electron Participation in Bonding
Several factors influence which electrons participate in chemical bonding:
- Electronegativity: Atoms with higher electronegativity tend to attract electrons more strongly, influencing the nature of the bond formed (ionic, polar covalent, or nonpolar covalent).
- Ionization energy: The energy required to remove an electron from an atom. Atoms with lower ionization energies readily lose electrons, often forming ionic bonds.
- Electron affinity: The energy change associated with the addition of an electron to an atom. Atoms with high electron affinities readily gain electrons, often forming ionic bonds or participating in covalent bonds where they attract electrons more strongly.
- Atomic size: Larger atoms generally have valence electrons further from the nucleus, making them more readily available for bonding.
Advanced Concepts and Exceptions
While the valence electrons are primarily involved in bonding, some exceptions and nuances exist:
- Coordinate covalent bonds (dative bonds): In these bonds, both electrons shared in the bond originate from the same atom. This type of bond is common in coordination complexes.
- Expanded octets: Certain elements in the third period and beyond can accommodate more than eight electrons in their valence shell, exceeding the octet rule. This is due to the availability of empty d orbitals. Examples include phosphorus pentachloride (PCl₅) and sulfur hexafluoride (SF₆).
- Incomplete octets: Some atoms, particularly those of elements in the second period (like beryllium and boron), can form stable compounds with fewer than eight valence electrons.
Conclusion
Chemical bonding is a complex process governed by the behavior of electrons, primarily valence electrons. Understanding the role of these electrons in different bonding types—ionic, covalent, and metallic—is essential for predicting the properties and reactivity of substances. While the principles outlined here offer a robust foundation, exceptions and more complex scenarios warrant further investigation for a deeper understanding of the fascinating world of chemical bonding. The interplay of electronegativity, ionization energy, electron affinity, and atomic size significantly impacts how electrons are involved in the formation of chemical bonds, leading to the diversity of chemical compounds we observe in nature and create in the laboratory. The exploration of this topic continues to be a vibrant area of research in chemistry.
Latest Posts
Latest Posts
-
Under What Conditions Are Gases Most Likely To Behave Ideally
Apr 02, 2025
-
How To Find The Vertical Asymptote Of A Limit
Apr 02, 2025
-
Confidence Interval Calculator For 2 Proportions
Apr 02, 2025
-
Draw A Structural Formula For 3 Bromo 4 Chloro 1 1 Dimethylcyclohexane
Apr 02, 2025
-
What Is A Primary Standard Chemistry
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Which Electrons Are Involved In Chemical Bonding . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
