Which Functional Groups Are Present In All Amino Acids
Muz Play
Mar 19, 2025 · 7 min read
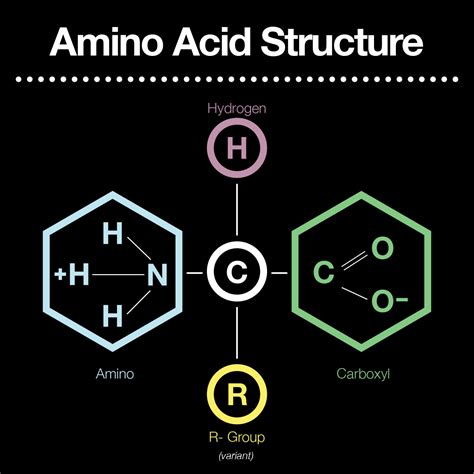
Table of Contents
Which Functional Groups are Present in All Amino Acids?
Amino acids, the fundamental building blocks of proteins, share a common core structure characterized by the presence of specific functional groups. Understanding these functional groups is crucial for comprehending the diverse properties and functions of amino acids and the proteins they form. This article delves into the essential functional groups found in all amino acids, exploring their chemical properties and biological significance. We'll also touch upon the variations that exist within these groups and their impact on amino acid behavior.
The Core Structure: A Universal Framework
All amino acids, with the exception of proline (which has a unique cyclic structure), share a basic structural framework consisting of:
-
A central carbon atom (α-carbon): This carbon atom is chiral (except for glycine, where it's achiral) and acts as the central point of attachment for the other functional groups.
-
An amino group (-NH₂): This is a basic functional group, capable of accepting a proton (H⁺) to become positively charged (-NH₃⁺) at physiological pH. Its basicity is a key factor in amino acid interactions and protein folding. The pKa of the amino group is typically around 9.0-10.0, meaning at physiological pH (around 7.4), it exists predominantly in its protonated form.
-
A carboxyl group (-COOH): This is an acidic functional group, capable of donating a proton (H⁺) to become negatively charged (-COO⁻) at physiological pH. Its acidity is vital for amino acid interactions and peptide bond formation. The pKa of the carboxyl group is typically around 2.0-3.0, meaning at physiological pH, it exists predominantly in its deprotonated form.
-
A hydrogen atom (-H): This simple group completes the tetrahedral arrangement around the α-carbon.
-
A side chain (R-group): This is the variable component that distinguishes one amino acid from another. The R-group can be anything from a simple hydrogen atom (as in glycine) to complex aromatic rings (as in tryptophan) or sulfur-containing chains (as in cysteine). The properties of the R-group, including its size, charge, polarity, and hydrophobicity, significantly influence the amino acid's behavior and its role in protein structure and function.
The Amino and Carboxyl Groups: The Pillars of Amino Acid Chemistry
The amino (-NH₂) and carboxyl (-COOH) groups are arguably the most important functional groups in all amino acids. Their presence defines the very nature of amino acids as zwitterions.
Zwitterions: The Dual Nature of Amino Acids
At physiological pH, amino acids exist as zwitterions. This means they carry both a positive charge (on the amino group) and a negative charge (on the carboxyl group) within the same molecule. This bipolar nature is responsible for many of the unique properties of amino acids, including their high melting points and solubility in water. The zwitterionic form is crucial for their interaction with other molecules and their role in protein structure.
Peptide Bond Formation: Linking Amino Acids
The carboxyl group of one amino acid and the amino group of another amino acid can react to form a peptide bond through a dehydration reaction (loss of a water molecule). This reaction is fundamental for protein synthesis, where amino acids are linked together to form polypeptide chains. The peptide bond is a crucial element in the primary structure of proteins, dictating their sequence of amino acids.
The Side Chain (R-group): The Source of Diversity
While the amino and carboxyl groups are common to all amino acids, the side chain (R-group) is unique to each amino acid, contributing to its individual chemical properties and biological functions. R-groups are broadly classified based on their properties:
1. Nonpolar, Aliphatic Side Chains: Hydrophobic Interactions
These side chains are predominantly composed of hydrocarbons and are hydrophobic (water-repelling). Examples include:
- Glycine (Gly, G): The simplest amino acid, with a single hydrogen atom as its side chain.
- Alanine (Ala, A): Contains a methyl group (-CH₃) as its side chain.
- Valine (Val, V): Has a branched isopropyl group (-CH(CH₃)₂) as its side chain.
- Leucine (Leu, L): Features an isobutyl group (-CH₂CH(CH₃)₂) as its side chain.
- Isoleucine (Ile, I): Has a branched sec-butyl group (-CH(CH₂CH₃)CH₃) as its side chain.
- Methionine (Met, M): Contains a thioether group (-CH₂CH₂SCH₃) in its side chain.
These hydrophobic side chains play a crucial role in protein folding, often clustering together in the interior of proteins to minimize contact with water.
2. Aromatic Side Chains: Absorption and Interactions
These side chains contain aromatic rings, contributing to their unique properties:
- Phenylalanine (Phe, F): Contains a benzene ring as its side chain.
- Tyrosine (Tyr, Y): Has a benzene ring with a hydroxyl group (-OH) attached.
- Tryptophan (Trp, W): Features an indole ring as its side chain.
Aromatic side chains can participate in various interactions, including hydrophobic interactions, hydrogen bonding (in the case of tyrosine), and π-π stacking interactions. They often play important roles in protein-protein interactions and enzyme activity.
3. Polar, Uncharged Side Chains: Hydrogen Bonding and Interactions
These side chains contain polar functional groups that can participate in hydrogen bonding:
- Serine (Ser, S): Contains a hydroxyl group (-OH) as its side chain.
- Threonine (Thr, T): Has a hydroxyl group (-OH) attached to a β-carbon.
- Cysteine (Cys, C): Contains a sulfhydryl group (-SH) as its side chain. This group is capable of forming disulfide bonds, crucial for protein structure stabilization.
- Asparagine (Asn, N): Contains a carboxamide group (-CONH₂) as its side chain.
- Glutamine (Gln, Q): Has a carboxamide group (-CONH₂) attached to a longer chain.
These polar side chains are often found on the surface of proteins, interacting with water molecules and other polar molecules.
4. Positively Charged Side Chains (Basic): Electrostatic Interactions
These side chains are positively charged at physiological pH:
- Lysine (Lys, K): Contains an amino group (-NH₃⁺) at the end of its side chain.
- Arginine (Arg, R): Features a guanidinium group as its side chain.
- Histidine (His, H): Has an imidazole ring as its side chain.
The positive charges on these side chains can participate in electrostatic interactions with negatively charged molecules or groups. Histidine's unique pKa (around 6.0) allows it to act as a buffer, accepting or donating protons near physiological pH.
5. Negatively Charged Side Chains (Acidic): Electrostatic Interactions
These side chains are negatively charged at physiological pH:
- Aspartic Acid (Asp, D): Contains a carboxyl group (-COO⁻) as its side chain.
- Glutamic Acid (Glu, E): Has a carboxyl group (-COO⁻) attached to a longer chain.
These negatively charged side chains participate in electrostatic interactions, often interacting with positively charged molecules or groups.
The Significance of Functional Groups in Protein Structure and Function
The presence and arrangement of these functional groups in amino acids are crucial for determining the final structure and function of proteins. The interactions between these groups drive the folding of polypeptide chains into specific three-dimensional structures (secondary, tertiary, and quaternary structures).
-
Hydrogen bonding: The amino, carboxyl, and polar side chains frequently participate in hydrogen bonding, stabilizing secondary structures like α-helices and β-sheets.
-
Hydrophobic interactions: Nonpolar side chains cluster together in the protein's interior, minimizing their contact with water and contributing to protein stability.
-
Electrostatic interactions: Positively and negatively charged side chains can interact through electrostatic forces, further stabilizing the protein's structure.
-
Disulfide bonds: The sulfhydryl groups of cysteine residues can form covalent disulfide bonds, creating strong cross-links within the protein and contributing significantly to its stability.
The specific arrangement of these functional groups determines the protein's overall shape and, consequently, its function. Enzymes, for example, rely on specific arrangements of amino acid side chains within their active sites to bind substrates and catalyze reactions. Antibodies utilize specific combinations of functional groups to recognize and bind to foreign antigens. Structural proteins such as collagen depend on specific amino acid interactions to provide strength and support.
Conclusion: A Unified Framework for Biological Diversity
All amino acids, despite their diversity in side chains, share a common core structure defined by the presence of an amino group, a carboxyl group, a hydrogen atom, and a central α-carbon. This common core provides a unified framework for understanding the diverse properties and functions of these building blocks of life. The remarkable diversity arises from the unique properties of each amino acid's side chain, enabling the formation of proteins with an extraordinary range of structures and functions crucial for all living organisms. Understanding the interplay of these functional groups is therefore essential for a comprehensive grasp of biochemistry, molecular biology, and the very essence of life itself.
Latest Posts
Latest Posts
-
Eriksons Stages Of Development Integrity Vs Despair
Mar 19, 2025
-
Is Stearic Acid Polar Or Nonpolar
Mar 19, 2025
-
Antimicrobial Sensitivity Testing Kirby Bauer Method
Mar 19, 2025
-
Ir Spectrum Of A Carboxylic Acid
Mar 19, 2025
-
Which Statement About Thylakoids In Eukaryotes Is Not Correct
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Which Functional Groups Are Present In All Amino Acids . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
