Ir Spectrum Of A Carboxylic Acid
Muz Play
Mar 19, 2025 · 6 min read
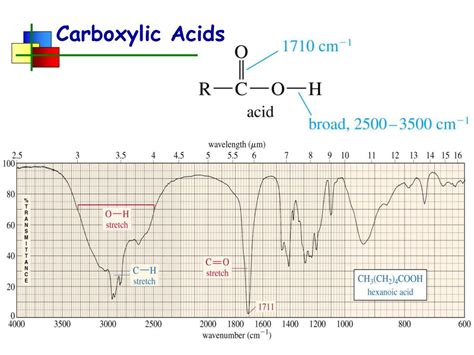
Table of Contents
Infrared Spectroscopy of Carboxylic Acids: A Comprehensive Guide
Infrared (IR) spectroscopy is a powerful analytical technique used to identify functional groups within a molecule. The specific absorption patterns observed in an IR spectrum provide a unique "fingerprint" for a compound, allowing for its identification and characterization. Carboxylic acids, with their characteristic carboxyl group (-COOH), exhibit distinct IR absorption bands that are crucial for their identification and structural elucidation. This article delves into the intricacies of the IR spectrum of carboxylic acids, exploring the various vibrational modes and their corresponding absorption frequencies. We will also discuss factors that can influence the spectral features and how to interpret the data effectively.
Understanding the Carboxyl Group and its Vibrational Modes
The carboxyl group (-COOH) is comprised of a hydroxyl group (-OH) and a carbonyl group (C=O) bonded to the same carbon atom. This arrangement results in several distinct vibrational modes that contribute to the unique IR spectrum of carboxylic acids. These vibrational modes include:
1. O-H Stretching Vibration
The O-H stretching vibration is typically observed as a broad, intense band in the region of 2500-3300 cm⁻¹. The broadness is a characteristic feature of carboxylic acids and is due to hydrogen bonding between the hydroxyl groups of neighboring molecules. The strong hydrogen bonding causes variations in the O-H bond length and strength, leading to a wider range of absorption frequencies. The exact position of this band can vary slightly depending on the solvent and the degree of hydrogen bonding. The presence of this broad, intense band in this region is a strong indication of the presence of a carboxylic acid functional group.
2. C=O Stretching Vibration
The carbonyl group (C=O) in carboxylic acids exhibits a strong stretching vibration, typically appearing as a sharp, intense band in the region of 1680-1725 cm⁻¹. The precise frequency of this band depends on factors such as the nature of the substituents on the molecule, the solvent, and the degree of hydrogen bonding. In general, the C=O stretching frequency in carboxylic acids is slightly lower than that observed in ketones or aldehydes due to the influence of the hydroxyl group. This strong absorption band is a key diagnostic feature for identifying the presence of a carbonyl group within the molecule.
3. O-H Bending Vibration
The O-H bending vibration, often referred to as the "in-plane" bending, is observed as a medium intensity band usually around 900-1300 cm⁻¹. The exact position and intensity of this band can be somewhat variable and might be overlapped by other absorptions, making it less reliable for identification compared to the O-H and C=O stretching vibrations.
4. C-O Stretching Vibration
The C-O stretching vibration of the carboxyl group is usually a medium intensity band found in the region of 1200-1320 cm⁻¹. This band arises from the stretching of the carbon-oxygen single bond within the carboxyl group. Like the O-H bending vibration, it can be less distinct and potentially obscured by other absorptions.
Factors Affecting the IR Spectrum of Carboxylic Acids
Several factors can influence the appearance and precise frequencies of the absorption bands in the IR spectrum of a carboxylic acid:
-
Hydrogen Bonding: The most significant factor influencing the IR spectrum of carboxylic acids is hydrogen bonding. The strong intermolecular hydrogen bonding between carboxylic acid molecules significantly affects the O-H stretching frequency, causing the broadening effect previously discussed. In highly diluted solutions or in the gas phase, where hydrogen bonding is minimized, the O-H stretching band becomes narrower and shifts to higher wavenumbers.
-
Solvent Effects: The solvent used to prepare the sample can also influence the position and shape of the absorption bands. Polar solvents can increase the extent of hydrogen bonding, leading to further broadening of the O-H stretching band and slight shifts in the other absorption frequencies.
-
Substituent Effects: The presence of electron-donating or electron-withdrawing groups on the carbon atom adjacent to the carboxyl group can affect the electron density within the carboxyl group, influencing the stretching frequencies of the C=O and C-O bonds. Electron-withdrawing groups tend to shift the C=O stretching frequency to higher wavenumbers, while electron-donating groups have the opposite effect.
-
Concentration: The concentration of the carboxylic acid in the sample can affect the intensity of the absorption bands. Higher concentrations generally lead to more intense bands, particularly the O-H stretching band due to increased hydrogen bonding.
-
Sample Preparation: The method used to prepare the sample for IR spectroscopy can also influence the resulting spectrum. For example, the use of a potassium bromide (KBr) pellet technique can lead to different results compared to using a solution cell, due to variations in intermolecular interactions.
Interpreting the IR Spectrum of Carboxylic Acids: A Practical Approach
Interpreting the IR spectrum of a carboxylic acid involves a systematic approach focusing on the key diagnostic bands:
-
Look for the broad, intense absorption band between 2500-3300 cm⁻¹: This is the hallmark of the O-H stretching vibration and provides strong evidence for the presence of a carboxylic acid group. The broadness is a crucial characteristic.
-
Identify the strong, sharp band in the region of 1680-1725 cm⁻¹: This is the C=O stretching vibration, further confirming the presence of the carboxyl group.
-
Examine the region around 1200-1320 cm⁻¹ for the C-O stretching vibration: This band is less prominent but can provide additional support for the identification.
-
Consider the influence of other functional groups: The presence of other functional groups in the molecule can influence the position and shape of the carboxyl group absorption bands. Careful examination of the entire spectrum is essential for a complete interpretation.
-
Compare to reference spectra: Comparing the obtained spectrum to reference spectra of known carboxylic acids can be valuable for confirming the identification and assessing the purity of the sample. Spectra databases and literature are helpful resources for this comparison.
Advanced Considerations and Applications
The IR spectroscopy of carboxylic acids extends beyond the basic identification of the functional group. Advanced techniques and interpretations can provide further insights:
-
Deuteration Studies: Replacing the hydroxyl hydrogen with deuterium (²H) can provide valuable information on the O-H vibrational modes, as the O-D stretching frequency is shifted to lower wavenumbers.
-
Matrix Isolation Spectroscopy: This technique involves isolating the carboxylic acid molecules in a low-temperature matrix, minimizing intermolecular interactions and providing more accurate information on the vibrational modes.
-
Computational Spectroscopy: Computational methods can be used to predict the IR spectrum of carboxylic acids, aiding in the assignment of vibrational modes and providing further insights into the molecular structure and dynamics.
Conclusion
Infrared spectroscopy provides a powerful and efficient method for the identification and characterization of carboxylic acids. The characteristic broad O-H stretching band and the strong C=O stretching band are key diagnostic features. Understanding the influence of various factors, such as hydrogen bonding and solvent effects, is crucial for accurate interpretation of the spectra. Combining IR spectroscopy with other analytical techniques, such as NMR and mass spectrometry, provides a comprehensive approach to structural elucidation. The ongoing advancements in IR spectroscopy and computational methods continue to enhance the capabilities of this invaluable analytical tool in the study of carboxylic acids and other organic molecules. This in-depth understanding of the IR spectrum of carboxylic acids allows for its effective application across various fields including organic chemistry, biochemistry, and materials science.
Latest Posts
Latest Posts
-
An Atom That Has Gained Or Lost Electrons
Mar 20, 2025
-
Compounds Containing Only Carbon And Hydrogen Are Called
Mar 20, 2025
-
How Many Covalent Bonds Does Hydrogen Have
Mar 20, 2025
-
Oxidation Of An Aldehyde Produces A
Mar 20, 2025
-
Evidence That Light Is A Particle
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Ir Spectrum Of A Carboxylic Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
