Is Stearic Acid Polar Or Nonpolar
Muz Play
Mar 19, 2025 · 6 min read
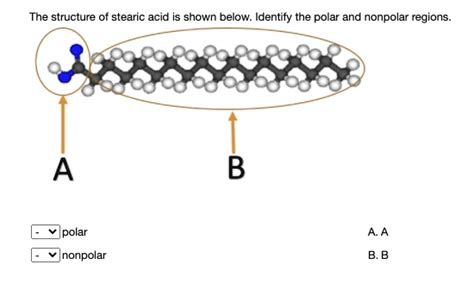
Table of Contents
Is Stearic Acid Polar or Nonpolar? Understanding the Properties of a Common Fatty Acid
Stearic acid, a saturated fatty acid prevalent in many animal and vegetable fats, often sparks curiosity among chemistry enthusiasts and students alike. A common question that arises is: Is stearic acid polar or nonpolar? The answer isn't a simple yes or no, and understanding the nuances requires delving into the molecular structure and its interactions with other molecules. This comprehensive guide will explore the polarity of stearic acid, its implications, and its relevance in various applications.
Understanding Polarity and Nonpolarity
Before diving into the specifics of stearic acid, it's crucial to grasp the fundamental concepts of polarity and nonpolarity. Polarity arises from the unequal sharing of electrons in a covalent bond. This unequal sharing happens when one atom in the bond is more electronegative than the other, attracting the shared electrons more strongly. This creates a dipole moment, with one end of the molecule having a partial negative charge (δ-) and the other end having a partial positive charge (δ+). Water (H₂O) is a classic example of a polar molecule.
Nonpolar molecules, on the other hand, involve the relatively equal sharing of electrons between atoms in a covalent bond. The electronegativity difference between the atoms is minimal, leading to no significant charge separation. Examples of nonpolar molecules include methane (CH₄) and many hydrocarbons.
The Structure of Stearic Acid: A Long Carbon Chain
Stearic acid (CH₃(CH₂)₁₆COOH) boasts a long, unbranched hydrocarbon chain consisting of 18 carbon atoms. The vast majority of this molecule is composed of carbon-carbon (C-C) and carbon-hydrogen (C-H) bonds. These bonds are considered nonpolar because carbon and hydrogen have very similar electronegativities. The difference in electronegativity is so small that the electron distribution is nearly equal.
However, stearic acid also possesses a carboxyl group (-COOH) at one end. This carboxyl group is the key to understanding the nuances of stearic acid's polarity. The oxygen atoms in the carboxyl group are significantly more electronegative than the carbon and hydrogen atoms. This electronegativity difference results in a polar region within the molecule. The C=O bond is highly polar due to a large difference in electronegativity between carbon and oxygen, while the O-H bond is also polar due to the significant electronegativity difference between oxygen and hydrogen. This leads to the formation of a dipole moment within the carboxyl group.
The Predominantly Nonpolar Nature of Stearic Acid
Despite the presence of the polar carboxyl group, the long nonpolar hydrocarbon chain significantly overshadows the polar end in terms of its overall influence on the molecule's behavior. This is why stearic acid is often classified as predominantly nonpolar. The hydrocarbon chain's length contributes significantly to this. The larger the hydrocarbon chain, the more the polar head is dominated by the nonpolar tail in terms of influencing the overall properties. This is analogous to a small, lightweight weight attached to one end of a long, heavy rope – the rope's properties will largely determine the overall behavior of the system.
Solubility and Polarity: A Key Indicator
Solubility provides a crucial indicator of a substance's polarity. "Like dissolves like" is a fundamental principle in chemistry: polar solvents dissolve polar solutes, and nonpolar solvents dissolve nonpolar solutes.
Stearic acid exhibits low solubility in polar solvents like water. The long hydrocarbon chain resists interaction with water molecules, which are strongly polar and form hydrogen bonds. While the carboxyl group can potentially form hydrogen bonds with water, it's insufficient to overcome the hydrophobic nature of the hydrocarbon chain. Conversely, stearic acid exhibits higher solubility in nonpolar solvents like hexane and benzene. This confirms its predominantly nonpolar character.
Implications of Stearic Acid's Predominantly Nonpolar Nature
The predominantly nonpolar nature of stearic acid significantly impacts its properties and applications:
1. Fatty Acid Behavior: Part of Lipids & Membranes
Stearic acid's nonpolar nature is pivotal to its role as a building block for lipids and cell membranes. The hydrophobic hydrocarbon chain allows it to aggregate with other fatty acids, forming hydrophobic interactions that are crucial for the structure and function of cell membranes. These interactions help to maintain the integrity of the cell membrane, forming a barrier between the internal and external cellular environments.
2. Industrial Applications: Cosmetics and Candles
Stearic acid's properties find use in various industrial applications. Its nonpolarity contributes to its use in cosmetics as an emulsifier and thickener. It is also used in the production of candles due to its ability to solidify into a hard, stable mass. The nonpolar nature ensures it doesn't readily interact with water, maintaining the integrity of the candle.
3. Soap Making: A Subtle Shift in Polarity
While predominantly nonpolar, the carboxyl group of stearic acid undergoes saponification, a process that transforms it into soap. Saponification involves reacting stearic acid with a strong base, typically sodium hydroxide (NaOH). This reaction forms a carboxylate salt, which contains a charged carboxylate group (-COO⁻). The introduction of this charged group significantly alters the polarity of the molecule, making it amphipathic – possessing both polar and nonpolar regions. This amphipathic nature is crucial to the cleansing properties of soap, allowing it to interact with both polar (water) and nonpolar (oil and grease) substances.
4. Food Industry: Stabilizer & Emulsifier
Stearic acid finds application in the food industry as a stabilizer and emulsifier, owing to its ability to interact with both polar and nonpolar compounds. Although predominantly nonpolar, its limited polarity plays a role in its emulsifying capabilities, aiding in the mixing of ingredients with differing polarities. This is particularly useful in the production of processed foods.
Amphipathic Nature: A Further Nuance
It is crucial to highlight that while stearic acid is predominantly nonpolar, it's more accurate to describe it as amphipathic rather than simply "polar" or "nonpolar". This amphipathic nature refers to its possession of both hydrophilic (water-loving) and hydrophobic (water-fearing) regions. The carboxyl group represents the hydrophilic region, while the long hydrocarbon chain constitutes the hydrophobic region. This duality in character is what allows stearic acid to play its vital roles in various biological and industrial processes.
Conclusion: A Detailed Perspective
The question of whether stearic acid is polar or nonpolar demands a nuanced answer. While possessing a polar carboxyl group, the long nonpolar hydrocarbon chain makes stearic acid predominantly nonpolar. Its amphipathic nature, however, is a crucial aspect of its behavior and functionality, influencing its solubility, interactions with other molecules, and ultimately, its varied applications in biology and industry. Understanding this duality is vital for appreciating the multifaceted nature of this common fatty acid and its significance in a range of contexts. Further exploration into the effects of chain length and the influence of other functional groups on the overall polarity of fatty acids would provide a more comprehensive understanding of this fascinating class of molecules.
Latest Posts
Latest Posts
-
An Atom That Has Gained Or Lost Electrons
Mar 20, 2025
-
Compounds Containing Only Carbon And Hydrogen Are Called
Mar 20, 2025
-
How Many Covalent Bonds Does Hydrogen Have
Mar 20, 2025
-
Oxidation Of An Aldehyde Produces A
Mar 20, 2025
-
Evidence That Light Is A Particle
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Is Stearic Acid Polar Or Nonpolar . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
