Which Is A Property Of Ionic Compounds
Muz Play
Mar 29, 2025 · 5 min read
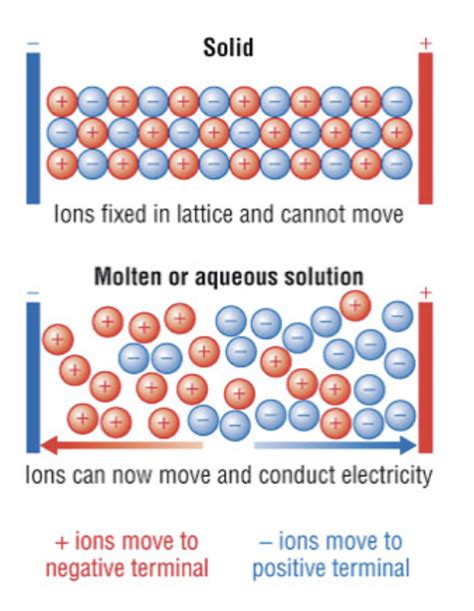
Table of Contents
Properties of Ionic Compounds: A Deep Dive
Ionic compounds, formed through the electrostatic attraction between oppositely charged ions, exhibit a unique set of properties that distinguish them from other types of compounds like covalent compounds. Understanding these properties is crucial in various fields, from chemistry and materials science to geology and biology. This comprehensive guide delves into the key characteristics of ionic compounds, explaining the underlying reasons for their behavior.
Key Properties of Ionic Compounds
Several key properties define ionic compounds:
1. High Melting and Boiling Points:
Ionic compounds possess remarkably high melting and boiling points. This stems from the strong electrostatic forces of attraction between the oppositely charged ions. To melt or boil an ionic compound, a significant amount of energy is required to overcome these strong interactions and break the crystalline lattice structure. The stronger the electrostatic forces (influenced by the charges of the ions and the distance between them), the higher the melting and boiling points will be. For example, sodium chloride (NaCl) has a high melting point of 801°C, reflecting the strength of the ionic bonds within its crystal lattice.
2. Crystalline Structure:
Ionic compounds typically exist as crystalline solids at room temperature. This ordered arrangement maximizes the electrostatic attraction between ions of opposite charges. The ions are arranged in a regular, repeating three-dimensional pattern called a crystal lattice. The specific arrangement depends on the size and charge of the ions involved. This crystalline structure contributes to their characteristic hardness and brittleness.
3. Hardness and Brittleness:
Ionic compounds are generally hard but brittle. The hardness arises from the strong electrostatic forces holding the ions in the crystal lattice. However, their brittleness is a consequence of the rigid arrangement of ions. When subjected to stress, the layers of ions can shift, bringing ions of like charges into close proximity. This results in strong repulsive forces that cause the crystal to fracture easily along specific cleavage planes.
4. Electrical Conductivity:
Ionic compounds are typically poor conductors of electricity in their solid state. This is because the ions are held rigidly in the crystal lattice and are unable to move freely to carry an electric current. However, ionic compounds become good conductors when molten (liquid) or dissolved in water (aqueous solution). In these states, the ions are free to move, allowing them to conduct electricity. This conductivity is directly related to the movement of charged particles.
5. Solubility:
The solubility of ionic compounds varies widely depending on the specific compound and the solvent. Many ionic compounds are soluble in polar solvents like water. This is because water molecules, being polar, can interact with and surround the ions, effectively separating them from the crystal lattice through a process called hydration. The interaction between the ions and water molecules is stronger than the ionic bonds within the crystal, leading to dissolution. However, ionic compounds are generally insoluble in nonpolar solvents.
6. Water Solutions Conduct Electricity:
As mentioned earlier, ionic compounds dissolved in water form aqueous solutions that conduct electricity. This is because the water molecules separate the ions, allowing them to move freely and carry an electric current. The conductivity of the solution depends on the concentration of dissolved ions; higher concentrations lead to higher conductivity. This principle is the basis for many applications, including batteries and electroplating.
Factors Influencing the Properties of Ionic Compounds
Several factors influence the specific properties of ionic compounds:
1. Charge of Ions:
The magnitude of the charges on the ions significantly affects the strength of the electrostatic forces. Higher charges lead to stronger attractions and thus higher melting points, greater hardness, and lower solubility. For instance, magnesium oxide (MgO), with Mg²⁺ and O²⁻ ions, has a much higher melting point than sodium chloride (NaCl), with Na⁺ and Cl⁻ ions.
2. Size of Ions:
The size of the ions also plays a critical role. Smaller ions can pack more closely together, resulting in stronger electrostatic forces and higher melting points. Conversely, larger ions are farther apart, leading to weaker attractions and lower melting points.
3. Lattice Energy:
Lattice energy is a measure of the energy released when ions come together to form a crystal lattice. A higher lattice energy indicates stronger ionic bonds, which in turn leads to higher melting points, greater hardness, and lower solubility. The lattice energy is directly proportional to the product of the ionic charges and inversely proportional to the distance between the ions.
4. Polarity of the Solvent:
As previously discussed, the polarity of the solvent greatly influences the solubility of ionic compounds. Polar solvents like water can effectively dissolve ionic compounds, whereas nonpolar solvents cannot. This difference arises from the ability of polar solvents to interact with and stabilize the ions through dipole-dipole interactions.
Examples of Ionic Compounds and Their Applications
Ionic compounds are ubiquitous in nature and have numerous applications:
- Sodium chloride (NaCl): Table salt, essential for human health and used extensively in various industries.
- Calcium carbonate (CaCO₃): A major component of limestone and marble, used in construction and as a source of calcium.
- Sodium hydroxide (NaOH): A strong base used in various industrial processes, including soap making and paper production.
- Potassium permanganate (KMnO₄): A strong oxidizing agent used as a disinfectant and in chemical synthesis.
- Many minerals: Numerous minerals are ionic compounds, playing vital roles in the Earth's geology and contributing to various resources.
Conclusion
Ionic compounds are characterized by a unique set of properties arising from the strong electrostatic forces between oppositely charged ions. Their high melting points, crystalline structure, hardness, brittleness, electrical conductivity in molten or aqueous states, and solubility in polar solvents are all direct consequences of the ionic bonding. Understanding these properties is essential for a wide range of scientific and technological applications. The specific properties of individual ionic compounds are further influenced by factors such as the size and charge of the ions and the lattice energy of the crystal. This knowledge provides a fundamental framework for comprehending the behavior and applications of a vast array of materials in our daily lives and various industries.
Latest Posts
Latest Posts
-
The Amount Of Matter In An Object Is
Apr 01, 2025
-
What Is The Amdr For Fat For Adults
Apr 01, 2025
-
What Is The Difference Between A Closed And Open System
Apr 01, 2025
-
What Is The Charge Of Sodium
Apr 01, 2025
-
What Is Primary Standard In Chemistry
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Which Is A Property Of Ionic Compounds . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
