Which Orbital Has The Highest Energy
Muz Play
Mar 25, 2025 · 6 min read
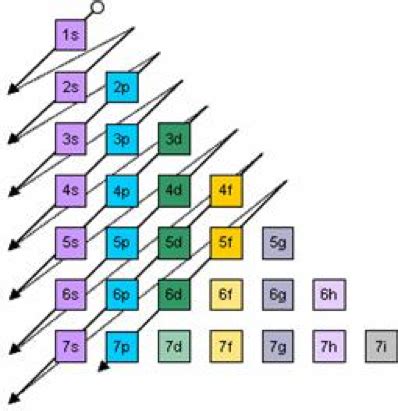
Table of Contents
Which Orbital Has the Highest Energy? A Deep Dive into Atomic Structure
Understanding which atomic orbital possesses the highest energy is fundamental to comprehending the behavior of atoms and molecules. It's not a simple "one size fits all" answer, as the energy levels of orbitals are influenced by several interacting factors. This article will delve into the intricacies of atomic orbitals, exploring the factors that determine orbital energy and ultimately answering the question of which orbital holds the highest energy.
The Quantum Mechanical Model and Atomic Orbitals
Before we dive into orbital energies, let's establish a basic understanding of the quantum mechanical model of the atom. This model depicts electrons not as orbiting particles like planets around the sun, but as existing in regions of space called atomic orbitals. These orbitals are defined by solutions to the Schrödinger equation, a complex mathematical equation that describes the behavior of electrons in an atom. The solutions yield a set of quantum numbers that characterize each orbital:
-
Principal Quantum Number (n): This number determines the energy level and size of the orbital. Higher values of n indicate higher energy levels and larger orbitals. n can be any positive integer (1, 2, 3, etc.).
-
Azimuthal Quantum Number (l): This number determines the shape of the orbital and its angular momentum. For a given n, l can range from 0 to n - 1. l = 0 corresponds to an s orbital (spherical), l = 1 corresponds to p orbitals (dumbbell-shaped), l = 2 corresponds to d orbitals (more complex shapes), and so on.
-
Magnetic Quantum Number (ml): This number specifies the orientation of the orbital in space. For a given l, ml can range from -l to +l, including 0. For example, for l = 1 (p orbitals), ml can be -1, 0, or +1, representing three p orbitals oriented along the x, y, and z axes.
-
Spin Quantum Number (ms): This number describes the intrinsic angular momentum (spin) of the electron. It can have a value of +1/2 or -1/2. Each orbital can hold a maximum of two electrons with opposite spins (Pauli Exclusion Principle).
Factors Affecting Orbital Energy
The energy of an atomic orbital is primarily determined by the principal quantum number (n). Higher values of n correspond to higher energy levels. However, the story doesn't end there. Several other factors subtly influence orbital energies, leading to complexities in the ordering of energy levels:
1. The Principal Quantum Number (n): The Dominant Factor
As mentioned earlier, n is the most significant factor determining orbital energy. Orbitals with higher n values are further from the nucleus and experience less attraction from the positively charged protons. This results in higher energy levels. For example, a 3s orbital is higher in energy than a 2s orbital, and a 4s orbital is higher than a 3s orbital.
2. The Azimuthal Quantum Number (l): Penetration and Shielding
While n is the dominant factor, the azimuthal quantum number (l) also plays a role. Orbitals with the same n but different l values have slightly different energies due to penetration and shielding.
-
Penetration: This refers to the ability of an electron in a particular orbital to penetrate closer to the nucleus. s orbitals penetrate more effectively than p orbitals, which in turn penetrate more effectively than d orbitals, and so on. Greater penetration leads to a stronger attraction to the nucleus and a lower energy.
-
Shielding: Inner electrons shield outer electrons from the full positive charge of the nucleus. This shielding effect reduces the effective nuclear charge experienced by outer electrons, increasing their energy. s orbitals are less shielded than p orbitals, which are less shielded than d orbitals.
The interplay between penetration and shielding explains why, for example, a 2s orbital is slightly lower in energy than a 2p orbital, even though they have the same n value. The 2s orbital penetrates closer to the nucleus, experiencing a greater effective nuclear charge and lower energy.
3. Electron-Electron Repulsion: A Complicating Factor
In atoms with multiple electrons, electron-electron repulsion becomes a significant factor influencing orbital energies. Electrons repel each other due to their like charges. This repulsion increases the energy of the orbitals and can affect the ordering of energy levels, especially in larger atoms.
4. Effective Nuclear Charge: The Net Attraction
The effective nuclear charge (Z<sub>eff</sub>) is the net positive charge experienced by an electron after accounting for shielding effects. It is a crucial factor influencing orbital energy. A higher Z<sub>eff</sub> means a stronger attraction to the nucleus and lower orbital energy. The shielding effect of inner electrons reduces Z<sub>eff</sub> for outer electrons.
Determining the Highest Energy Orbital: A Case-by-Case Approach
There isn't a single definitive answer to "which orbital has the highest energy?" The answer depends on the specific atom and its electronic configuration. However, we can make some general observations:
-
For a given atom: The highest energy occupied orbital is the one with the highest n value in its valence shell. This is the outermost occupied shell.
-
Across the periodic table: As we move across a period (from left to right), the effective nuclear charge increases, leading to a decrease in the energy of the orbitals. However, the addition of electrons also leads to increased electron-electron repulsion which can partially counteract this.
-
Down the periodic table: As we move down a group, the principal quantum number increases, resulting in a significant increase in the energy of the orbitals. The increasing number of inner electrons also leads to increased shielding, partially mitigating the increase in nuclear charge.
Examples:
-
Hydrogen (H): The highest energy occupied orbital is the 1s orbital.
-
Helium (He): The highest energy occupied orbital is also the 1s orbital, but it's lower in energy compared to the 1s in Hydrogen due to the higher effective nuclear charge.
-
Lithium (Li): The highest energy occupied orbital is the 2s orbital.
-
Oxygen (O): The highest energy occupied orbitals are the 2p orbitals.
-
Larger atoms: For larger atoms with many electrons, the energy ordering of orbitals becomes more complex due to the increasing influence of electron-electron repulsion. The relative energies of orbitals such as 4s, 3d, and 4p can vary depending on the specific atom. Generally, the (n+1)s orbital will be lower in energy than the nd orbital for atoms in the third and subsequent periods.
The Role of Spectroscopic Data and Calculations
Experimental techniques like photoelectron spectroscopy provide valuable data on the ionization energies of electrons in different orbitals. This data allows us to determine the relative energies of the orbitals experimentally. Furthermore, sophisticated computational methods, such as Density Functional Theory (DFT) and Hartree-Fock calculations, provide theoretical tools to calculate the energies of atomic and molecular orbitals with high accuracy. These computational methods can handle the complexities of electron-electron interactions and provide accurate estimations of orbital energies even for complex systems.
Conclusion
Determining the highest energy orbital is a nuanced question, dependent on the atom in question and the interplay of several quantum mechanical factors. While the principal quantum number (n) provides a first-order approximation, penetration, shielding, electron-electron repulsion, and effective nuclear charge all contribute significantly to the final energy level of an orbital. Understanding these factors is crucial for interpreting atomic and molecular behavior and predicting chemical properties. The relative energies of orbitals are best determined through a combination of experimental techniques and theoretical calculations, providing a comprehensive picture of atomic structure and electronic configuration. This detailed understanding is fundamental to numerous areas within chemistry, physics, and materials science.
Latest Posts
Latest Posts
-
What Happens To A Plant Cell In Hypertonic Solution
Mar 26, 2025
-
Atomic Mass Equals The Number Of
Mar 26, 2025
-
Plot A Normal Distribution In R
Mar 26, 2025
-
Place The Steps Of Eukaryotic Transcription In Order Of Occurrence
Mar 26, 2025
-
Where Is The Mass Of The Atom Located
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Which Orbital Has The Highest Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
