Which Part Of An Amino Acid Is Always Acidic
Muz Play
Mar 30, 2025 · 5 min read
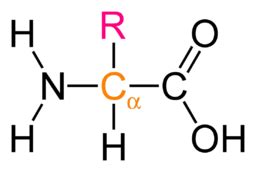
Table of Contents
Which Part of an Amino Acid is Always Acidic? Understanding the Carboxyl Group
Amino acids, the fundamental building blocks of proteins, possess a unique structure that dictates their function and properties. While the diversity of amino acids arises from variations in their side chains (R groups), one part of their structure remains consistently acidic: the carboxyl group (-COOH). This article delves deep into the chemistry of the carboxyl group, its acidic nature, its role in peptide bond formation, and its significance in various biological processes.
The Structure of an Amino Acid: A Closer Look
Before focusing on the acidic carboxyl group, it's crucial to understand the basic structure of an amino acid. Every amino acid, with few exceptions, shares a common core structure:
- A central carbon atom (α-carbon): This carbon atom is chiral (except for glycine), meaning it has four different groups attached to it.
- An amino group (-NH₂): This group is basic, capable of accepting a proton (H⁺).
- A carboxyl group (-COOH): This is the group we'll be focusing on – it's acidic, capable of donating a proton.
- A side chain (R group): This is the variable part of the amino acid, differing in structure and properties across the 20 standard amino acids. It's the R group that largely determines the unique characteristics of each amino acid.
The Carboxyl Group: The Source of Acidity
The carboxyl group (-COOH) is the functional group responsible for the acidic nature of amino acids. Its acidity stems from the polarity of the C=O bond and the ability of the hydroxyl group (-OH) to readily release a proton.
Understanding Acid-Base Chemistry
Acidity is defined by the ability of a molecule to donate a proton (H⁺). A strong acid readily donates its proton, while a weak acid donates it less readily. The carboxyl group acts as a weak acid. The process of proton donation is depicted below:
R-COOH ⇌ R-COO⁻ + H⁺
In this equilibrium, the carboxyl group (R-COOH) donates a proton (H⁺) to form a carboxylate ion (R-COO⁻). The equilibrium lies slightly to the left, indicating that the carboxyl group is a relatively weak acid. However, even this weak acidity plays a vital role in many biological processes.
Resonance Stabilization of the Carboxylate Ion
The stability of the carboxylate ion (R-COO⁻) is crucial to its formation. The negative charge on the carboxylate ion is delocalized across both oxygen atoms due to resonance. This delocalization distributes the negative charge, making the ion more stable than it would be if the charge were localized on a single oxygen atom. This resonance stabilization significantly contributes to the carboxyl group's ability to release a proton.
The pKa Value and its Significance
The acidity of a molecule is quantitatively represented by its pKa value. The pKa is the pH at which half of the molecules are protonated and half are deprotonated. The carboxyl group of amino acids typically has a pKa value around 2. This means that at a physiological pH of 7.4, the carboxyl group is almost entirely deprotonated (exists as R-COO⁻). This deprotonated form plays a critical role in peptide bond formation.
Peptide Bond Formation: The Role of the Carboxyl Group
One of the most crucial functions of the carboxyl group is its participation in peptide bond formation. Peptide bonds link amino acids together to form polypeptide chains, the precursors to proteins.
The formation of a peptide bond involves a dehydration reaction between the carboxyl group of one amino acid and the amino group of another. The carboxyl group donates its hydroxyl group (-OH) as a water molecule, and the remaining carboxyl carbon forms a covalent bond with the nitrogen atom of the adjacent amino acid's amino group.
This process creates a peptide bond (-CO-NH-), a crucial link in the polypeptide chain. The carboxyl group's ability to lose a hydroxyl group, facilitated by its acidic nature, is absolutely essential to this fundamental process in protein synthesis.
Beyond Peptide Bonds: Other Biological Roles of the Carboxyl Group
The acidic carboxyl group, beyond its role in peptide bond formation, participates in several other critical biological processes:
- Enzyme Catalysis: Many enzymes utilize carboxyl groups in their active sites to catalyze reactions. The ability to donate or accept protons is crucial for acid-base catalysis.
- Protein-Protein Interactions: Charged carboxyl groups can participate in electrostatic interactions with other charged or polar groups in proteins, influencing protein folding, stability, and interactions.
- Metal Ion Binding: The carboxylate ion (R-COO⁻) can bind to metal ions, often playing a role in the function of metalloproteins.
- Regulation of Enzyme Activity: The protonation state of the carboxyl group can be affected by pH changes, acting as a regulatory mechanism for enzyme activity.
- Post-Translational Modifications: Carboxyl groups can undergo various post-translational modifications, such as amidation, impacting protein function.
The Exceptional Case of Glycine
While the carboxyl group is consistently acidic in all amino acids, it's important to briefly mention glycine. Glycine is unique among the 20 standard amino acids because its side chain is simply a hydrogen atom. This lack of a significant side chain means that glycine's properties are largely determined by its amino and carboxyl groups. However, the acidic nature of the carboxyl group remains consistent even in this simplest amino acid.
Conclusion: The Central Role of the Acidic Carboxyl Group
The carboxyl group (-COOH) is undeniably the always acidic part of an amino acid. Its acidic nature, driven by the polarity of the carbonyl group and the resonance stabilization of the resulting carboxylate ion, is fundamental to the amino acid's function. From the creation of peptide bonds, which form the backbone of proteins, to its participation in a wide array of biological processes such as enzyme catalysis, metal binding, and protein-protein interactions, the carboxyl group’s consistently acidic behavior serves as a cornerstone of life's molecular machinery. Understanding the chemistry of this vital functional group provides essential insights into the complexity and elegance of biological systems. This inherent acidity, coupled with the diverse nature of the side chains, allows for the vast array of protein structures and functions found in living organisms. The subtle differences in pKa values among various amino acids further fine-tune their behavior within complex biological contexts. The carboxyl group's contribution is not merely about acidity; it’s about the precise chemical potential it offers, essential for life as we know it.
Latest Posts
Latest Posts
-
Cross Section Of A Woody Stem
Apr 01, 2025
-
A State Function Is Best Described As
Apr 01, 2025
-
What Is The Electron Configuration For Ne
Apr 01, 2025
-
Difference Between E1 And E2 Reaction
Apr 01, 2025
-
Journal Entry To Issue Common Stock
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Which Part Of An Amino Acid Is Always Acidic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
