Which Sample Of Matter Is Classified As A Solution
Muz Play
Mar 15, 2025 · 6 min read
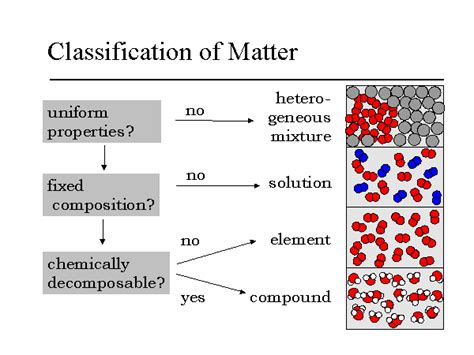
Table of Contents
Which Sample of Matter is Classified as a Solution? A Deep Dive into Solution Chemistry
Understanding the classification of matter is fundamental to chemistry. While elements, compounds, and mixtures represent broad categories, mixtures themselves are further subdivided based on their properties. One crucial type of mixture is a solution, a homogeneous mixture with unique characteristics that distinguish it from other types of mixtures like suspensions and colloids. This article delves deep into what constitutes a solution, exploring its defining properties, providing numerous examples, and differentiating it from other mixtures.
Defining a Solution: Homogeneity and Particle Size
A solution is defined as a homogeneous mixture of two or more substances. The key to understanding this definition lies in the word "homogeneous." This means that the mixture has a uniform composition throughout. No matter where you sample the solution, you'll find the same proportion of its components. This is in stark contrast to heterogeneous mixtures, where the components are visibly distinct, like sand in water.
The uniformity of a solution is directly related to the particle size of the components. In a solution, the particles of the solute (the substance being dissolved) are incredibly small, typically on the order of nanometers (10<sup>-9</sup> meters). These particles are completely dispersed and individually surrounded by particles of the solvent (the substance doing the dissolving). This incredibly small particle size is the reason solutions appear clear and transparent, unlike suspensions which are cloudy.
Key Components of a Solution: Solute and Solvent
Every solution contains two essential components:
-
Solute: This is the substance that is dissolved in the solvent. It's usually present in a smaller amount than the solvent. Examples include sugar in sugar water, salt in saltwater, and oxygen in air.
-
Solvent: This is the substance that dissolves the solute. It's typically the component present in the larger amount. Water is the most common solvent, often referred to as the "universal solvent" due to its ability to dissolve a wide range of substances. Other common solvents include ethanol, acetone, and benzene.
Types of Solutions: Beyond the Basics
While water-based solutions are common, solutions can exist in various phases:
-
Solid Solutions: These are formed when one solid is dissolved in another. Examples include alloys like brass (copper and zinc) and steel (iron and carbon). These solid solutions are often created through melting and then cooling the mixture, allowing the atoms to uniformly mix.
-
Liquid Solutions: These are solutions where the solvent is a liquid. This is the most common type of solution, encompassing examples like saltwater, sugar water, and many chemical solutions used in laboratories.
-
Gaseous Solutions: Gases can also form solutions, where one gas is dissolved in another. Air is a prime example, being a solution of nitrogen, oxygen, argon, and other gases.
Differentiating Solutions from other Mixtures: Suspensions and Colloids
It's crucial to distinguish solutions from other types of mixtures, particularly suspensions and colloids. These mixtures differ in particle size and whether they are homogeneous or heterogeneous:
-
Suspensions: These are heterogeneous mixtures with larger particles than solutions. The particles will settle out over time if left undisturbed. Examples include muddy water or sand in water. The particles in a suspension are easily visible.
-
Colloids: Colloids represent an intermediate state between solutions and suspensions. They have larger particles than solutions but smaller than suspensions. The particles are dispersed but do not settle out over time. They often exhibit the Tyndall effect, scattering light, making them appear cloudy or opaque. Examples include milk, fog, and ink.
Factors Affecting Solubility: Concentration and Saturation
The extent to which a solute dissolves in a solvent is its solubility. This solubility is influenced by several factors:
-
Temperature: Solubility often increases with increasing temperature for solids and liquids dissolved in liquids. However, the solubility of gases in liquids generally decreases with increasing temperature.
-
Pressure: Pressure has a significant effect on the solubility of gases in liquids. Higher pressure leads to greater solubility, as seen in carbonated beverages, where high pressure forces carbon dioxide into the solution.
-
Nature of the Solute and Solvent: The chemical properties of the solute and solvent dictate their compatibility. "Like dissolves like" is a common principle: polar solvents (like water) dissolve polar solutes, while nonpolar solvents (like oil) dissolve nonpolar solutes.
The concentration of a solution refers to the amount of solute dissolved in a given amount of solvent or solution. A solution can be described as:
-
Unsaturated: When more solute can be dissolved at a given temperature and pressure.
-
Saturated: When the maximum amount of solute has been dissolved at a given temperature and pressure. Adding more solute will not dissolve; it will remain as a precipitate.
-
Supersaturated: A solution containing more solute than it can theoretically hold at a given temperature and pressure. These are unstable and can easily precipitate the excess solute.
Real-World Examples of Solutions: A Diverse Range
Solutions are ubiquitous in our daily lives and in numerous industrial processes. Here are some diverse examples:
-
Seawater: A solution of salt (primarily sodium chloride) and various other minerals dissolved in water.
-
Sugar Water: A simple solution of sugar (sucrose) dissolved in water.
-
Air: A gaseous solution of primarily nitrogen and oxygen, with trace amounts of other gases.
-
Brass: A solid solution of copper and zinc, forming a strong and malleable alloy.
-
Vinegar: A solution of acetic acid in water.
-
Gasoline: A solution of various hydrocarbons.
-
Many pharmaceutical drugs: Many medications are solutions or suspensions of active ingredients in a suitable solvent for easy administration.
Applications of Solutions in Various Fields: A Broad Spectrum
The properties of solutions make them indispensable across various fields:
-
Medicine: Solutions are used to deliver drugs intravenously, orally, or topically. Intravenous solutions maintain fluid balance and deliver essential electrolytes.
-
Industry: Solutions are vital in manufacturing processes, such as electroplating, etching, and cleaning. Solutions are also essential in the production of many chemicals and materials.
-
Agriculture: Solutions of fertilizers provide essential nutrients to plants.
-
Environmental Science: The solubility of pollutants in water is crucial in understanding environmental contamination and remediation.
-
Food Science: Many food products are solutions or suspensions, including sauces, beverages, and dressings.
Conclusion: Understanding Solutions for a Deeper Appreciation of Chemistry
The concept of solutions is a cornerstone of chemistry, impacting our daily lives in countless ways. By understanding the definition, properties, and diverse examples of solutions, we can better appreciate their importance and the fundamental principles of homogeneous mixtures. The ability to differentiate solutions from suspensions and colloids emphasizes the importance of particle size and homogeneity in classifying matter. Furthermore, considering the factors that influence solubility allows us to predict and control the behavior of solutions in various applications. This comprehensive understanding is key to further exploration in diverse scientific and engineering fields.
Latest Posts
Latest Posts
-
How To Find A Perpendicular Vector
Mar 15, 2025
-
How Could Sulfur Form An Ion
Mar 15, 2025
-
What Elemsnts Are Most Likey To Turn Into Anions Why
Mar 15, 2025
-
What Is The Difference Between Hunger And Appetite
Mar 15, 2025
-
Boiling Point On Graph In Celsius
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Which Sample Of Matter Is Classified As A Solution . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
