Which Substance Is An Arrhenius Base
Muz Play
Mar 24, 2025 · 6 min read
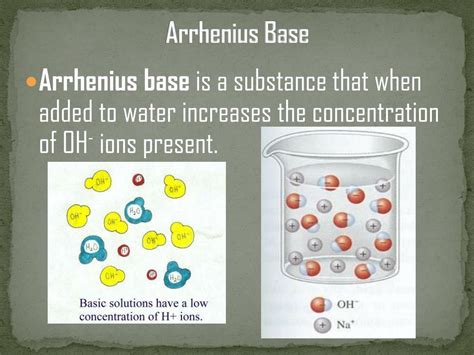
Table of Contents
Which Substance is an Arrhenius Base? A Comprehensive Guide
Understanding Arrhenius bases is fundamental to grasping acid-base chemistry. This comprehensive guide delves deep into the definition, properties, examples, and applications of Arrhenius bases, equipping you with a robust understanding of this crucial concept. We'll explore how to identify Arrhenius bases and differentiate them from other types of bases, clarifying common misconceptions along the way.
Defining an Arrhenius Base
The cornerstone of understanding Arrhenius bases lies in their precise definition: an Arrhenius base is a substance that increases the concentration of hydroxide ions (OH⁻) when dissolved in water. This seemingly simple definition encompasses a wide range of compounds and reactions, and it's crucial to remember the context of aqueous solutions. The presence of water is essential; the dissociation and subsequent increase in hydroxide ions wouldn't occur in other solvents.
Key Characteristics of Arrhenius Bases:
- Hydroxide Ion Production: The defining characteristic is the production of OH⁻ ions upon dissolution in water. This is the litmus test for identifying an Arrhenius base.
- Aqueous Solutions: The reaction must take place in water. The interaction between the base and water is crucial for the dissociation process.
- pH Greater Than 7: Arrhenius bases, in aqueous solutions, result in a pH value greater than 7, indicating alkalinity. The higher the pH, the stronger the base.
- Reaction with Acids: They readily react with Arrhenius acids, neutralizing each other in a process that produces salt and water (neutralization reaction).
Examples of Common Arrhenius Bases
Let's examine some common substances that fit the definition of an Arrhenius base:
1. Metal Hydroxides:
These are perhaps the most straightforward examples. Metal hydroxides, such as sodium hydroxide (NaOH), potassium hydroxide (KOH), calcium hydroxide (Ca(OH)₂), and magnesium hydroxide (Mg(OH)₂), readily dissociate in water, releasing hydroxide ions.
Example: NaOH(s) → Na⁺(aq) + OH⁻(aq)
The dissolution of sodium hydroxide in water produces sodium cations (Na⁺) and hydroxide anions (OH⁻), thus increasing the hydroxide ion concentration and exhibiting the behavior of an Arrhenius base. The strength of these bases varies; NaOH and KOH are strong bases, completely dissociating in water, while Ca(OH)₂ and Mg(OH)₂ are weaker bases.
2. Alkali Metal Hydroxides:
Alkali metals (Group 1 elements) form hydroxides that are all strong Arrhenius bases. These readily dissociate completely into their constituent ions in water. This complete dissociation leads to a high concentration of hydroxide ions, resulting in a highly alkaline solution.
3. Alkaline Earth Metal Hydroxides:
Alkaline earth metals (Group 2 elements) also form hydroxides, but these are generally weaker bases than alkali metal hydroxides. They don't dissociate completely in water, leading to a lower concentration of hydroxide ions.
4. Some Metal Oxides:
Certain metal oxides, particularly those of alkali and alkaline earth metals, react with water to form metal hydroxides, which then act as Arrhenius bases. This is an indirect method of increasing hydroxide ion concentration.
Example: Na₂O(s) + H₂O(l) → 2NaOH(aq)
Sodium oxide reacts vigorously with water to produce sodium hydroxide, a strong Arrhenius base.
Differentiating Arrhenius Bases from Other Base Types
While the Arrhenius definition is straightforward, it's important to distinguish it from broader definitions of bases, like Brønsted-Lowry and Lewis bases.
Arrhenius vs. Brønsted-Lowry Bases:
The Brønsted-Lowry definition is more expansive. A Brønsted-Lowry base is a proton (H⁺) acceptor. All Arrhenius bases are also Brønsted-Lowry bases because the hydroxide ion can accept a proton. However, not all Brønsted-Lowry bases are Arrhenius bases. Ammonia (NH₃), for instance, is a Brønsted-Lowry base because it accepts a proton, but it doesn't directly increase the hydroxide ion concentration in water like an Arrhenius base. It increases the OH⁻ concentration indirectly through a reaction with water.
Arrhenius vs. Lewis Bases:
The Lewis definition is the most general. A Lewis base is an electron-pair donor. This definition encompasses a much broader range of substances than Arrhenius or Brønsted-Lowry bases. Many compounds can donate electron pairs without necessarily increasing the hydroxide ion concentration in water or accepting protons.
In summary: The Arrhenius definition is the most restrictive, with Brønsted-Lowry being more inclusive, and Lewis being the most general. All Arrhenius bases are Brønsted-Lowry bases, and all Brønsted-Lowry bases are Lewis bases, but the reverse is not true.
Applications of Arrhenius Bases
Arrhenius bases have numerous practical applications across various fields:
1. Industrial Applications:
- Soap and Detergents: Many soaps and detergents are produced using strong Arrhenius bases like NaOH and KOH in a process called saponification.
- Paper Production: Arrhenius bases are crucial in the pulping process of paper manufacturing, breaking down lignin in wood.
- Textile Industry: Bases are used in textile dyeing and processing.
- Chemical Synthesis: Many chemical reactions rely on bases as catalysts or reactants.
2. Everyday Applications:
- Drain Cleaners: Many drain cleaners utilize strong bases to dissolve organic materials blocking drains.
- Antacids: Some antacids contain weak bases to neutralize stomach acid. However, these aren't strictly Arrhenius bases in their mode of action.
- Food Production: Certain food items utilize bases for pH control and preservation.
3. Laboratory Applications:
- Titrations: Arrhenius bases are commonly used in acid-base titrations to determine the concentration of acids.
- pH Control: Maintaining specific pH levels in reactions or solutions often requires the use of bases.
Identifying Arrhenius Bases: A Practical Approach
Identifying an Arrhenius base often involves a combination of observation and chemical knowledge. Here's a step-by-step approach:
-
Check for Hydroxide Ions (OH⁻): The presence of hydroxide ions (OH⁻) in the chemical formula is a strong indicator. For example, NaOH, KOH, and Ca(OH)₂ clearly contain hydroxide ions.
-
Consider the Metal Cation: Alkali and alkaline earth metal hydroxides are generally Arrhenius bases. The greater the electropositivity of the metal, the stronger the base.
-
Examine the Reaction with Water: If a substance dissolves in water and increases the concentration of hydroxide ions, it is an Arrhenius base. This can often be determined experimentally by measuring the pH of the solution.
-
Consider the Metal Oxide Reaction: Some metal oxides react with water to form hydroxides, indirectly increasing the hydroxide ion concentration.
-
Consult a Chemistry Reference: Referencing reliable chemistry textbooks or databases can confirm whether a specific substance is an Arrhenius base.
Common Misconceptions about Arrhenius Bases
It's crucial to address common misconceptions surrounding Arrhenius bases:
- All Bases are Arrhenius Bases: This is incorrect. Many bases are not Arrhenius bases but fit the broader Brønsted-Lowry or Lewis definitions.
- Only Hydroxides are Bases: While metal hydroxides are common Arrhenius bases, other substances can also increase hydroxide ion concentration in water.
- All Arrhenius Bases are Strong: While many common examples are strong bases, some metal hydroxides are weaker bases.
Conclusion: A Deeper Understanding of Arrhenius Bases
Understanding Arrhenius bases is vital for mastering acid-base chemistry. This comprehensive guide has clarified the definition, properties, examples, and applications of Arrhenius bases, helping differentiate them from other base types and emphasizing the importance of their role in various chemical processes and industrial applications. By applying the practical approach outlined here, you can effectively identify Arrhenius bases and build a strong foundation in this essential area of chemistry. Remember, the key is the direct production of hydroxide ions (OH⁻) upon dissolution in water.
Latest Posts
Latest Posts
-
What Is The Average Kinetic Energy
Mar 25, 2025
-
What Are The Building Blocks Of Macromolecules
Mar 25, 2025
-
Chemistry The Molecular Nature Of Matter And Change 8th Edition
Mar 25, 2025
-
Which Event Always Involves A Chemical Change
Mar 25, 2025
-
What Happens When An Atom Gains An Electron
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Which Substance Is An Arrhenius Base . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
