Why Are More Substituted Alkenes More Stable
Muz Play
Mar 30, 2025 · 5 min read
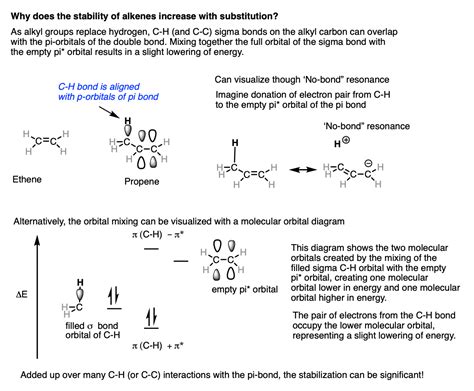
Table of Contents
Why Are More Substituted Alkenes More Stable? Understanding Hyperconjugation and Steric Effects
Alkenes, hydrocarbons containing a carbon-carbon double bond, exhibit a fascinating relationship between their stability and the number of alkyl groups attached to the double-bonded carbons. This phenomenon, where more substituted alkenes are more stable than less substituted alkenes, is a fundamental concept in organic chemistry. Understanding this stability difference requires a deep dive into the intricacies of hyperconjugation and steric effects.
The Hierarchy of Alkene Stability
Before delving into the underlying mechanisms, let's establish the clear hierarchy of alkene stability:
- Tetrasubstituted alkenes: These alkenes have four alkyl groups attached to the double-bonded carbons. They are the most stable.
- Trisubstituted alkenes: Possessing three alkyl groups attached to the double bond, these are the second most stable.
- Disubstituted alkenes: With two alkyl groups attached to the double bond, they exhibit intermediate stability.
- Monosubstituted alkenes: Having only one alkyl group attached to the double bond, these are less stable than disubstituted, trisubstituted, and tetrasubstituted alkenes.
- Un-substituted alkenes (ethylene): This is the least stable alkene, with only hydrogen atoms attached to the double-bonded carbons.
This stability order is consistently observed and has significant implications in predicting reaction outcomes and understanding reaction mechanisms.
The Role of Hyperconjugation
The primary factor driving the increased stability of more substituted alkenes is hyperconjugation. This is a stabilizing interaction that occurs between a filled bonding orbital (typically a C-H σ bond) and an adjacent empty or partially filled non-bonding or anti-bonding orbital (in this case, the π* antibonding orbital of the alkene).
Understanding the Mechanism
Imagine a C-H σ bond on an alkyl group adjacent to the double bond. This σ bond has electron density. The empty π* antibonding orbital of the alkene can accept some of this electron density. This interaction, the overlap between the filled σ orbital and the empty π* orbital, is hyperconjugation. The more alkyl groups present, the more C-H σ bonds are available for hyperconjugation.
The Stabilizing Effect
Hyperconjugation stabilizes the alkene by delocalizing electron density. This delocalization lowers the overall energy of the molecule, making it more stable. The increased number of alkyl groups in more substituted alkenes provides a greater number of C-H σ bonds for hyperconjugation, leading to a greater degree of stabilization. This is why tetrasubstituted alkenes are the most stable. They benefit from the maximum number of hyperconjugative interactions.
Visualizing Hyperconjugation
It's helpful to visualize hyperconjugation as a partial donation of electron density from the C-H σ bond to the π* antibonding orbital. This interaction weakens the C-H bond slightly and strengthens the C=C bond, contributing to the overall stabilization.
The Influence of Steric Effects
While hyperconjugation is the dominant factor in explaining alkene stability, steric effects also play a role, albeit a less significant one compared to hyperconjugation.
Steric Hindrance
Steric hindrance arises from the repulsion between electron clouds of atoms or groups that are close together in space. In highly substituted alkenes, the alkyl groups are bulky and can experience steric repulsion. This repulsion slightly destabilizes the molecule.
Balancing Hyperconjugation and Steric Effects
The interplay between hyperconjugation and steric effects determines the overall stability of the alkene. In most cases, the stabilizing influence of hyperconjugation outweighs the destabilizing effect of steric hindrance. However, in extremely bulky alkenes, the steric strain can become more pronounced, somewhat diminishing the overall stability gain from hyperconjugation.
Experimental Evidence Supporting Alkene Stability
The increased stability of more substituted alkenes is consistently observed in various experimental scenarios, providing robust support for the hyperconjugation and steric effects explanations.
Heat of Hydrogenation
The heat of hydrogenation, the enthalpy change associated with the addition of hydrogen across the double bond, provides a direct measure of alkene stability. Less substituted alkenes have higher heats of hydrogenation, indicating they are less stable and release more energy upon hydrogenation. More substituted alkenes have lower heats of hydrogenation, reflecting their greater inherent stability.
Reaction Rates
The relative rates of reactions involving alkenes also reflect their stability. Reactions that involve breaking the C=C double bond, such as electrophilic addition, proceed more slowly with more substituted alkenes, consistent with their increased stability. The higher activation energy required to initiate the reaction is a direct consequence of their higher stability.
Thermodynamic Stability
Thermodynamic studies further corroborate the stability trend. More substituted alkenes are favored thermodynamically, meaning they have a lower free energy and are thus more stable under equilibrium conditions.
Applications and Implications
The understanding of alkene stability has widespread applications in various areas of organic chemistry:
- Predicting Reaction Outcomes: Knowing the relative stability of alkenes helps predict the major product formed in reactions where multiple alkene isomers are possible. The more stable alkene isomer is generally the major product.
- Designing Organic Synthesis: Synthetic chemists utilize this knowledge to design efficient synthetic routes that favor the formation of the desired alkene isomer.
- Understanding Reaction Mechanisms: The stability differences provide insights into the mechanisms of reactions involving alkenes, such as electrophilic additions and eliminations.
- Polymer Chemistry: The stability of different alkene monomers influences the properties and stability of the resulting polymers.
Conclusion: A Multifaceted Perspective
The greater stability of more substituted alkenes is a testament to the intricate interplay of electronic and steric factors within molecules. While hyperconjugation is the primary driving force behind this stability trend, steric effects play a secondary, albeit significant, role, particularly in highly substituted systems. The consistent experimental evidence supports this model, underscoring its importance in understanding and predicting the behavior of alkenes in various chemical contexts. The knowledge of alkene stability is fundamental to organic chemistry, offering invaluable insights into reaction mechanisms, product prediction, and synthetic pathway design. It's a concept that continues to be explored and refined, driving further advancements in our understanding of molecular behavior and reactivity.
Latest Posts
Latest Posts
-
Cross Section Of A Woody Stem
Apr 01, 2025
-
A State Function Is Best Described As
Apr 01, 2025
-
What Is The Electron Configuration For Ne
Apr 01, 2025
-
Difference Between E1 And E2 Reaction
Apr 01, 2025
-
Journal Entry To Issue Common Stock
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Why Are More Substituted Alkenes More Stable . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
