Why Do Buffers Resist Ph Change
Muz Play
Mar 30, 2025 · 6 min read
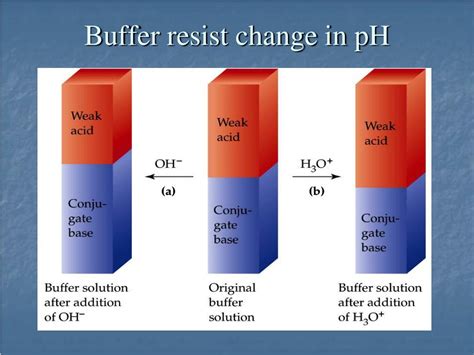
Table of Contents
Why Do Buffers Resist pH Change? A Deep Dive into Buffer Solutions
Buffers are solutions that resist changes in pH upon the addition of small amounts of acid or base. This crucial property makes them indispensable in various scientific fields, from biological systems to industrial processes. Understanding why buffers exhibit this resistance is fundamental to appreciating their significance. This article delves deep into the chemistry behind buffer action, exploring the different types of buffers, their applications, and the limitations of their buffering capacity.
The Chemistry of Buffering: The Hand-in-Hand Action of a Weak Acid and its Conjugate Base
The core principle behind buffer action lies in the equilibrium between a weak acid and its conjugate base (or a weak base and its conjugate acid). A weak acid is an acid that does not completely dissociate in water. Instead, it exists in equilibrium with its conjugate base and hydrogen ions (H⁺):
HA ⇌ H⁺ + A⁻
where:
- HA represents the weak acid
- H⁺ represents hydrogen ions
- A⁻ represents the conjugate base
This equilibrium is governed by the acid dissociation constant (Ka), which is a measure of the acid's strength. A lower Ka value indicates a weaker acid.
The key to understanding buffer resistance to pH change lies in Le Chatelier's principle. This principle states that if a change of condition is applied to a system in equilibrium, the system will shift in a direction that relieves the stress. Let's explore how this applies to buffer solutions:
Resisting the Addition of Acid
When a strong acid (like HCl) is added to a buffer solution containing a weak acid (HA) and its conjugate base (A⁻), the added H⁺ ions react with the conjugate base (A⁻):
H⁺ + A⁻ ⇌ HA
This reaction consumes the added H⁺ ions, preventing a significant decrease in pH. The equilibrium shifts to the right, favoring the formation of the weak acid HA. The change in pH is minimized because the added acid is consumed by the reaction with the conjugate base.
Resisting the Addition of Base
Similarly, when a strong base (like NaOH) is added to the buffer solution, the hydroxide ions (OH⁻) react with the weak acid (HA):
OH⁻ + HA ⇌ H₂O + A⁻
This reaction neutralizes the added OH⁻ ions, preventing a significant increase in pH. The equilibrium shifts to the right, favoring the formation of the conjugate base A⁻. Again, the change in pH is minimized because the added base is consumed by the reaction with the weak acid.
Types of Buffer Solutions
Different types of buffer solutions are used depending on the specific pH range required:
1. Acidic Buffers:
These buffers are used to maintain a pH below 7. Common examples include:
- Acetic acid/acetate buffer: This is a widely used buffer system composed of acetic acid (CH₃COOH) and its conjugate base, acetate (CH₃COO⁻).
- Phosphoric acid buffers: Phosphoric acid (H₃PO₄) has three dissociation constants, allowing the creation of buffers at different pH values.
2. Basic Buffers:
These buffers maintain a pH above 7. Examples include:
- Ammonia/ammonium buffer: This buffer system consists of ammonia (NH₃) and its conjugate acid, ammonium (NH₄⁺).
- Carbonate buffer: This system uses carbonic acid (H₂CO₃) and its conjugate bases, bicarbonate (HCO₃⁻) and carbonate (CO₃²⁻). This buffer is crucial in maintaining the pH of blood.
3. Physiological Buffers:
Living organisms rely heavily on buffer systems to maintain a stable internal environment. Crucial examples include:
- Phosphate buffer system: This system plays a critical role in maintaining the pH of intracellular fluids.
- Bicarbonate buffer system: This is the primary buffer system in blood, maintaining its pH around 7.4. It involves carbonic acid (H₂CO₃), bicarbonate (HCO₃⁻), and dissolved carbon dioxide (CO₂).
- Protein buffer system: Proteins, with their diverse amino acid side chains, contribute significantly to buffering capacity in biological systems.
The Henderson-Hasselbalch Equation: Quantifying Buffer Capacity
The Henderson-Hasselbalch equation provides a mathematical description of the relationship between the pH of a buffer solution and the concentrations of the weak acid and its conjugate base:
pH = pKa + log([A⁻]/[HA])
where:
- pH is the pH of the buffer solution
- pKa is the negative logarithm of the acid dissociation constant (Ka)
- [A⁻] is the concentration of the conjugate base
- [HA] is the concentration of the weak acid
This equation highlights that the pH of a buffer solution is determined by the ratio of the concentrations of the conjugate base and weak acid, as well as the pKa of the weak acid. A buffer is most effective when the concentrations of the weak acid and its conjugate base are approximately equal ([A⁻]/[HA] ≈ 1), resulting in a pH close to the pKa.
Buffer Capacity: The Limits of Resistance
While buffers resist pH changes, their ability to do so is not unlimited. Buffer capacity refers to the amount of acid or base that a buffer can neutralize before a significant change in pH occurs. Factors affecting buffer capacity include:
- Concentrations of the buffer components: Higher concentrations of the weak acid and its conjugate base lead to a greater buffer capacity.
- Ratio of weak acid to conjugate base: A buffer is most effective when the ratio of weak acid to conjugate base is close to 1.
- The pKa of the weak acid: The closer the pKa is to the desired pH, the more effective the buffer will be.
When the buffer capacity is exceeded, the addition of acid or base will result in a significant change in pH. This occurs when either the weak acid or the conjugate base is completely consumed by the added acid or base.
Applications of Buffer Solutions
The ability of buffers to resist pH change makes them essential in a wide range of applications:
1. Biological Systems:
- Maintaining blood pH: The bicarbonate buffer system plays a vital role in maintaining the pH of blood within a narrow range (7.35-7.45), which is crucial for proper physiological function.
- Enzyme activity: Many enzymes function optimally within a specific pH range. Buffers help maintain this pH, ensuring proper enzyme activity.
- Cell culture: Cell cultures require precise pH control for optimal growth and function. Buffers are used to maintain the desired pH in cell culture media.
2. Chemical and Industrial Processes:
- Calibration of pH meters: Buffer solutions of known pH are used to calibrate pH meters, ensuring accurate measurements.
- Analytical chemistry: Buffers are used in many analytical techniques to control pH and ensure the reproducibility of results.
- Electroplating: Buffers are used to control the pH of electroplating baths, maintaining consistent deposition rates and product quality.
- Food industry: Buffers help maintain the desired pH in food products, preventing spoilage and enhancing their quality.
- Pharmaceutical industry: Buffers are used in the formulation of drugs and pharmaceuticals to ensure stability and effectiveness.
3. Environmental Monitoring:
Buffers play a role in environmental monitoring, specifically in maintaining the pH of samples during analysis.
Conclusion: The Unsung Heroes of pH Stability
Buffers are indispensable tools in various fields, owing to their remarkable ability to resist changes in pH. Their ability to maintain a stable pH is crucial for countless biological processes and industrial applications. Understanding the chemistry behind buffer action, the factors affecting buffer capacity, and the diverse applications of buffer solutions is essential for anyone working in chemistry, biology, or related fields. By utilizing the principles of equilibrium, Le Chatelier's principle, and the Henderson-Hasselbalch equation, scientists and engineers can harness the power of buffers to achieve and maintain precise pH control in a wide variety of contexts.
Latest Posts
Latest Posts
-
What Does A Sigma Bond Look Like
Apr 01, 2025
-
What Are The Possible Offspring Genotypes
Apr 01, 2025
-
How To Calculate Voltage Of A Cell
Apr 01, 2025
-
What Are The Rights And Duties Of A Citizen
Apr 01, 2025
-
Criteria Of Good Measurement In Research
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Why Do Buffers Resist Ph Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
