2. Heat Transfer Through The Collision Of Molecules- Direct Contact
Muz Play
Mar 16, 2025 · 7 min read
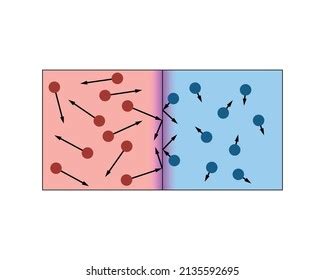
Table of Contents
Heat Transfer Through the Collision of Molecules: Direct Contact
Heat transfer, a fundamental concept in physics and engineering, describes the movement of thermal energy from a hotter region to a colder region. One of the primary mechanisms for this energy transfer is through the direct collision of molecules, a process often referred to as conduction. This article delves into the microscopic world to explore this phenomenon in detail, examining the factors influencing its efficiency and exploring its significance in various applications.
Understanding Molecular Collisions and Kinetic Energy
At the heart of heat transfer through direct contact lies the kinetic theory of matter. This theory postulates that all matter is composed of constantly moving molecules. These molecules possess kinetic energy, directly proportional to their temperature. A higher temperature implies a greater average kinetic energy of the constituent molecules.
When two objects at different temperatures come into contact, the faster-moving molecules in the hotter object collide with the slower-moving molecules in the colder object. These collisions transfer kinetic energy from the hotter molecules to the colder ones. This energy transfer continues until a thermal equilibrium is reached, where both objects are at the same temperature, and the average kinetic energy of molecules in both objects is equal.
The Role of Intermolecular Forces
The efficiency of this energy transfer through direct contact isn't solely determined by the difference in kinetic energies. The strength of intermolecular forces between the molecules also plays a crucial role. In solids, molecules are tightly bound together, facilitating efficient energy transfer through a chain reaction of collisions. This is why solids are generally good conductors of heat.
Liquids, with their weaker intermolecular forces and greater molecular mobility, exhibit intermediate heat conductivity. Gases, with their extremely weak intermolecular forces and large intermolecular distances, are poor conductors of heat, as the collisions between molecules are less frequent.
Factors Affecting Heat Transfer Through Direct Contact
Several factors influence the rate at which heat is transferred through direct contact:
1. Temperature Difference: The Driving Force
The temperature gradient, or the difference in temperature between the two objects, is the primary driving force behind heat transfer. A larger temperature difference leads to a higher rate of heat transfer, as the collisions between molecules become more energetic and frequent. This relationship is described by Fourier's Law of Heat Conduction, which states that the rate of heat transfer is directly proportional to the temperature gradient and the area of contact, and inversely proportional to the distance between the objects.
2. Material Properties: Thermal Conductivity
The thermal conductivity (k) of a material is a measure of its ability to conduct heat. Materials with high thermal conductivity, like metals (especially copper and silver), transfer heat efficiently due to the presence of free electrons that can readily transport energy. Conversely, materials with low thermal conductivity, like wood or insulating materials, are poor conductors of heat because their electrons are tightly bound to their atoms, restricting energy transfer.
Understanding thermal conductivity is crucial for material selection in various engineering applications. For instance, choosing materials with high thermal conductivity is essential in heat sinks designed to dissipate heat from electronic components. Conversely, materials with low thermal conductivity are vital for insulation in buildings to minimize energy loss.
3. Contact Area: Surface Area Matters
The larger the contact area between the two objects, the greater the number of molecular collisions, and therefore the faster the rate of heat transfer. This explains why spreading a hot substance over a larger area cools it down faster. Think of spreading hot sauce on a pizza – it cools quickly.
4. Contact Time: Duration of Interaction
The duration of contact between the two objects also affects heat transfer. Longer contact time allows for more molecular collisions, resulting in more efficient energy transfer. This is why, when cooking, you leave food on the stove for a certain period to ensure it's thoroughly heated.
5. Material Thickness: Distance Matters
The thickness of the material influences heat transfer, as a thicker material offers more resistance to heat flow. This is why thicker walls in buildings provide better insulation than thinner ones. Again, Fourier's Law emphasizes the inverse relationship between distance and the rate of heat transfer.
Applications of Heat Transfer Through Direct Contact
The principle of heat transfer through direct contact has widespread applications across various fields:
1. Cooking: From Stoves to Ovens
Cooking relies heavily on heat transfer through direct contact. Whether it's frying an egg in a pan or baking a cake in an oven, heat is transferred from the hot surface to the food, causing it to cook. The choice of cookware material (e.g., cast iron for even heat distribution) directly impacts the cooking process.
2. Heating and Cooling Systems: Radiators and Heat Sinks
Radiators in central heating systems and heat sinks in electronic devices utilize direct contact to transfer heat. The hot fluid (water or coolant) within the radiator or heat sink transfers its heat to the surrounding air or metal components through direct contact. The design of these systems carefully considers the material properties and surface area to optimize heat transfer efficiency.
3. Industrial Processes: Heat Exchangers
Heat exchangers are crucial in many industrial processes for transferring heat between fluids. These devices utilize direct contact between two fluids (or a fluid and a solid) to transfer heat effectively. The design of heat exchangers involves optimizing the surface area and material properties to achieve high efficiency.
4. Thermoregulation in Living Organisms: Maintaining Body Temperature
Living organisms maintain a stable internal body temperature through various mechanisms, and direct contact plays a crucial role. The circulatory system facilitates the transfer of heat from internal organs to the skin, which then dissipates the heat to the environment through direct contact with the air or water. The insulation provided by fat and fur minimizes heat loss through direct contact with the cold environment.
Advanced Concepts and Considerations
Beyond the basic principles, several advanced concepts contribute to a deeper understanding of heat transfer through direct contact:
1. Thermal Resistance: Opposing Heat Flow
Thermal resistance (R) is a measure of a material's ability to resist heat flow. A higher thermal resistance indicates that the material is a better insulator. It is the inverse of thermal conductance, which is related to thermal conductivity. Understanding thermal resistance is crucial for designing efficient insulation systems.
2. Thermal Diffusivity: How Quickly Heat Spreads
Thermal diffusivity (α) measures how quickly temperature changes propagate through a material. Materials with high thermal diffusivity experience rapid temperature changes, while materials with low thermal diffusivity exhibit slower temperature changes. This parameter is crucial in applications where rapid heating or cooling is required.
3. Boundary Conditions: Defining the System
Heat transfer problems are often defined by boundary conditions, which specify the temperature or heat flux at the boundaries of the system. These conditions significantly influence the heat transfer process. Different boundary conditions lead to different solutions in heat transfer calculations.
4. Transient Heat Transfer: Time-Dependent Processes
In many situations, heat transfer occurs over time. Transient heat transfer involves analyzing temperature changes within a system as a function of time. This type of analysis is necessary for situations where the temperature isn't constant, such as during the heating or cooling of an object.
Conclusion
Heat transfer through direct contact, driven by molecular collisions and governed by factors such as temperature difference, material properties, contact area, and time, is a fundamental phenomenon with significant implications in various fields. Understanding the microscopic mechanisms and macroscopic effects of this process is essential for designing efficient and effective systems in cooking, heating and cooling, industrial processes, and many other applications. Further research into advanced concepts like thermal resistance, diffusivity, and transient heat transfer deepens our understanding and allows for more precise engineering solutions. The continual exploration of this intricate process remains vital for advancements in diverse technological and scientific domains.
Latest Posts
Latest Posts
-
Circle Math Triangle Extending From Circle
Mar 17, 2025
-
According To The Kinetic Theory Of Gases
Mar 17, 2025
-
Do Valence Electrons Have The Most Energy
Mar 17, 2025
-
Element Vs Compound Vs Homogeneous Vs Heterogeneous
Mar 17, 2025
-
For An Exothermic Reaction The Products
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about 2. Heat Transfer Through The Collision Of Molecules- Direct Contact . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
