A Homogeneous Mixture Containing Particles That Scatter Light
Muz Play
Mar 16, 2025 · 6 min read
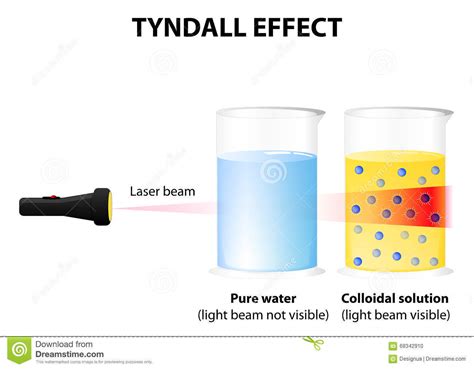
Table of Contents
A Homogeneous Mixture Containing Particles That Scatter Light: Delving into Colloidal Systems
A homogeneous mixture, by definition, presents a uniform composition throughout. However, the presence of particles within this mixture that scatter light introduces a fascinating layer of complexity. This phenomenon is not only visually striking but also holds significant scientific and technological implications. This article delves into the nature of these mixtures, focusing primarily on colloidal dispersions, where the suspended particles are of a size range that leads to significant light scattering.
Understanding Light Scattering in Homogeneous Mixtures
Light scattering, also known as scattering, is the phenomenon where light is forced to deviate from a straight trajectory due to interactions with particles. The extent and nature of this scattering are heavily dependent on several factors, including:
- Wavelength of light: Shorter wavelengths (e.g., blue light) are scattered more efficiently than longer wavelengths (e.g., red light). This is why the sky appears blue – shorter wavelengths are scattered more by atmospheric particles.
- Size of the scattering particles: The size of the particles relative to the wavelength of light plays a crucial role. Particles much smaller than the wavelength exhibit Rayleigh scattering, while larger particles lead to Mie scattering.
- Refractive index: The difference in refractive index between the particles and the surrounding medium significantly influences scattering intensity. A larger difference leads to stronger scattering.
- Particle concentration: Higher concentrations of scattering particles result in more intense scattering.
Rayleigh Scattering vs. Mie Scattering
Rayleigh scattering is dominant when the particle size is significantly smaller than the wavelength of light (typically less than 1/10th the wavelength). The scattered intensity is inversely proportional to the fourth power of the wavelength (I ∝ 1/λ⁴), hence the preference for shorter wavelengths. This scattering is symmetric, meaning it's equally intense in all directions.
Mie scattering, on the other hand, occurs when the particle size is comparable to or larger than the wavelength of light. This scattering is not as strongly dependent on wavelength and is highly asymmetric, with more light scattered in the forward direction. This is important in understanding phenomena like the appearance of clouds (which contain larger water droplets).
Colloidal Dispersions: The Perfect Example
Colloidal dispersions are homogeneous mixtures where the dispersed phase consists of particles with diameters ranging from 1 to 1000 nanometers. These particles are too large to be considered true solutions but are small enough to remain suspended in the medium without settling out due to Brownian motion (random thermal motion). This size range is ideal for significant light scattering, leading to the characteristic opalescence often observed in these systems.
Types of Colloidal Systems
Several types of colloidal systems exhibit light scattering:
- Sols: These are colloidal dispersions where the dispersed phase is solid particles suspended in a liquid medium. Examples include milk (fat globules in water), inks, and paints.
- Gels: These are colloidal systems where the dispersed phase forms a three-dimensional network throughout the continuous phase, resulting in a semi-solid structure. Examples include jelly and agar-agar.
- Emulsions: These are colloidal dispersions of two immiscible liquids, where one liquid is dispersed as droplets in the other. Examples include milk (water and fat), mayonnaise (oil and water), and some pharmaceutical preparations.
- Aerosols: These are colloidal dispersions of liquid or solid particles suspended in a gas. Examples include fog, clouds, and smoke.
Examples of Light Scattering in Colloidal Systems
Many everyday examples demonstrate light scattering in colloidal systems:
- Milk: The scattering of light by the fat globules in milk gives it its characteristic white appearance. If you shine a laser beam through milk, you'll observe a significant amount of scattered light.
- Opal: This gemstone owes its vibrant colors to the diffraction of light by regularly arranged silica spheres within its structure. The spacing between these spheres determines the wavelengths of light that are constructively interfered, giving rise to the characteristic iridescence.
- Clouds: Water droplets and ice crystals in clouds scatter sunlight, causing them to appear white or grey. The color and intensity depend on the size and concentration of the particles.
- Certain paints and inks: The pigments in these materials are colloidal particles that scatter light, contributing to their color and opacity.
Applications of Light Scattering in Colloidal Systems
The understanding and manipulation of light scattering in colloidal systems have far-reaching applications:
- Materials science: Light scattering techniques are employed to characterize the size, shape, and concentration of colloidal particles, aiding in the development of new materials with tailored optical properties.
- Biomedical applications: Light scattering is crucial in various biomedical techniques, such as flow cytometry (analyzing cells and particles in a fluid), particle size analysis in drug delivery systems, and studying biological tissues.
- Environmental science: Light scattering measurements help monitor air quality by detecting pollutants and aerosol particles in the atmosphere.
- Food science: Light scattering techniques are used to analyze the size and distribution of fat globules in milk and other food products.
- Chemical engineering: Light scattering is used to monitor and control the size and distribution of particles in various chemical processes.
Advanced Techniques for Studying Light Scattering
Several sophisticated techniques are used to study light scattering in colloidal systems:
- Dynamic Light Scattering (DLS): DLS measures the fluctuations in scattered light intensity caused by Brownian motion of the particles. This allows for the determination of particle size and diffusion coefficient.
- Static Light Scattering (SLS): SLS measures the time-averaged intensity of scattered light, providing information about particle size, shape, and molecular weight.
- Small Angle X-ray Scattering (SAXS): SAXS employs X-rays instead of visible light, enabling the study of even smaller particles and providing structural information about the colloidal system.
Conclusion: The Significance of Light Scattering
Light scattering in homogeneous mixtures, particularly colloidal dispersions, is a rich and complex phenomenon with widespread implications. The ability to understand and manipulate light scattering provides powerful tools for characterizing materials, analyzing biological systems, monitoring environmental quality, and developing new technologies. The ongoing research in this field continues to reveal new insights into the behavior of matter at the nanoscale and opens up exciting possibilities for future applications. From the iridescent beauty of an opal to the crucial role of light scattering in biomedical diagnostics, the study of these systems provides a fascinating journey into the interaction between light and matter. Further exploration into the intricacies of Rayleigh and Mie scattering and the development of increasingly sophisticated techniques will undoubtedly continue to shed light on this captivating field. The ongoing advancements in nanotechnology and materials science are further fueling this research, promising even more exciting discoveries and applications in the years to come. The implications extend to numerous sectors, making it a significant area of interdisciplinary research.
Latest Posts
Latest Posts
-
Circle Math Triangle Extending From Circle
Mar 17, 2025
-
According To The Kinetic Theory Of Gases
Mar 17, 2025
-
Do Valence Electrons Have The Most Energy
Mar 17, 2025
-
Element Vs Compound Vs Homogeneous Vs Heterogeneous
Mar 17, 2025
-
For An Exothermic Reaction The Products
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about A Homogeneous Mixture Containing Particles That Scatter Light . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
