Atoms Of The Same Element With Varying Number Of Neutrons.
Muz Play
Mar 17, 2025 · 7 min read
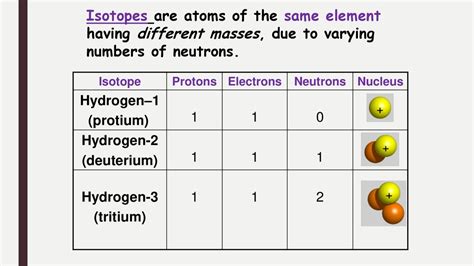
Table of Contents
Atoms of the Same Element with Varying Numbers of Neutrons: Isotopes and Their Significance
Atoms are the fundamental building blocks of matter, defining the properties of all substances. Each atom consists of a nucleus containing protons and neutrons, surrounded by a cloud of orbiting electrons. While the number of protons defines the element, the number of neutrons can vary, leading to the existence of isotopes. This article delves deep into the fascinating world of isotopes, exploring their properties, applications, and significance in various scientific fields.
Understanding Isotopes: A Deeper Dive
Isotopes are atoms of the same element that possess the same number of protons but differ in the number of neutrons. This difference in neutron number results in variations in their atomic mass. Since the number of protons dictates the element's identity (its atomic number), isotopes are variations of the same element. For example, carbon-12 (¹²C), carbon-13 (¹³C), and carbon-14 (¹⁴C) are all isotopes of carbon. They all have six protons, but they contain 6, 7, and 8 neutrons respectively. The superscript number represents the mass number (protons + neutrons).
Isotope Notation and Representation
Isotopes are typically represented using a specific notation. The notation includes the element's symbol, the mass number (A) as a superscript, and the atomic number (Z) as a subscript (although the atomic number is often omitted as it's implied by the element's symbol). For example:
- ¹²C: Carbon-12 (6 protons, 6 neutrons)
- ¹⁴N: Nitrogen-14 (7 protons, 7 neutrons)
- ²³⁵U: Uranium-235 (92 protons, 143 neutrons)
This notation clearly and concisely conveys the isotopic composition of an atom.
Properties of Isotopes: Similarities and Differences
While isotopes of the same element share the same number of protons and electrons, their differing neutron numbers lead to some key distinctions:
Similar Chemical Properties:
Because the number of electrons determines an element's chemical behavior, isotopes of the same element generally exhibit identical chemical properties. They react similarly in chemical reactions and form the same types of compounds. This similarity stems from the fact that chemical reactions involve the interaction of electrons in the outermost shell (valence electrons), which are unaffected by the varying neutron counts in the nucleus.
Different Physical Properties:
The differences in neutron numbers significantly impact the physical properties of isotopes. These differences manifest in several ways:
-
Mass: The most obvious difference is their mass. Heavier isotopes have greater mass due to the additional neutrons. This mass difference influences their rates of diffusion and effusion (movement of gases). Heavier isotopes diffuse and effuse more slowly than lighter ones.
-
Density: Density, which is mass per unit volume, is also affected. Heavier isotopes tend to have slightly higher densities than their lighter counterparts.
-
Nuclear Stability: The ratio of protons to neutrons significantly influences nuclear stability. Some isotopes are stable, meaning their nuclei do not spontaneously decay. Others are radioactive, meaning their nuclei are unstable and undergo radioactive decay, emitting particles or energy to achieve a more stable configuration.
-
Nuclear Spin: The number of neutrons influences the nuclear spin, a quantum mechanical property that affects the interaction of the nucleus with magnetic fields. This is crucial in techniques like nuclear magnetic resonance (NMR) spectroscopy.
Radioactive Isotopes and Their Applications
Radioactive isotopes, also known as radioisotopes, are unstable and undergo radioactive decay. This decay process emits various types of radiation, including alpha particles, beta particles, and gamma rays. The radiation emitted by radioisotopes has numerous applications in diverse fields:
Medical Applications:
-
Medical Imaging: Radioisotopes are widely used in medical imaging techniques like PET (Positron Emission Tomography) and SPECT (Single-Photon Emission Computed Tomography) scans. These techniques utilize radioisotopes that emit positrons or gamma rays, allowing physicians to visualize internal organs and detect abnormalities.
-
Radiation Therapy: Radioactive isotopes are employed in radiation therapy to target and destroy cancerous cells. Specific radioisotopes are incorporated into drugs or implants to deliver localized radiation to tumors.
-
Diagnostic Tests: Radioisotopes are used in various diagnostic tests to assess organ function and detect diseases. For example, iodine-131 is used to evaluate thyroid function.
Industrial Applications:
-
Radioactive Tracers: Radioisotopes are used as tracers to follow the movement of substances in industrial processes. For example, they can track the flow of fluids in pipelines or the wear and tear of machinery parts.
-
Sterilization: Gamma radiation from radioisotopes is used to sterilize medical equipment and food products. This method eliminates microorganisms without causing damage to the treated material.
-
Gauging and Measurement: Radioisotopes are employed in gauging techniques to measure the thickness of materials, level of liquids in containers, or density of substances.
Scientific Applications:
-
Radiocarbon Dating: Carbon-14, a radioactive isotope of carbon, is used to date organic materials. By measuring the remaining amount of carbon-14 in a sample, scientists can estimate its age. This technique is invaluable in archaeology, paleontology, and geology.
-
Nuclear Physics Research: Radioactive isotopes are essential tools for research in nuclear physics, providing insights into nuclear structure, decay processes, and interactions.
-
Geological Dating: Radioactive isotopes, like uranium and potassium, are used to date rocks and minerals. The decay rates of these isotopes allow scientists to determine the age of geological formations and the Earth itself.
Isotope Separation: Techniques and Challenges
The separation of isotopes is crucial for various applications, particularly in nuclear technology and medicine. However, it's a challenging task due to the similarity in chemical properties. Several techniques are used for isotope separation, each with its own advantages and limitations:
-
Gaseous Diffusion: This method exploits the slight mass difference between isotopes in gaseous form. Lighter isotopes diffuse faster through a porous membrane than heavier isotopes, leading to a partial separation.
-
Centrifugation: This technique uses high-speed centrifuges to separate isotopes based on their mass differences. Heavier isotopes tend to concentrate towards the outer edge of the centrifuge, while lighter isotopes remain closer to the center.
-
Laser Isotope Separation: Laser techniques utilize lasers tuned to specific wavelengths to selectively excite and ionize specific isotopes. This allows for efficient separation, especially for isotopes with small mass differences.
-
Electromagnetic Separation: This method utilizes strong magnetic and electric fields to deflect ions based on their mass-to-charge ratio. This technique is particularly effective for separating isotopes with larger mass differences.
The Significance of Isotopes in Various Scientific Disciplines
The study and application of isotopes have profoundly impacted many scientific fields:
Chemistry:
Isotopes provide valuable insights into reaction mechanisms, kinetics, and the structure of molecules. Isotopic labeling is commonly employed to trace the movement of atoms during chemical reactions.
Biology:
Isotopes are vital tools in biological research, particularly in studying metabolic processes, protein synthesis, and DNA replication. Radioactive isotopes are used as tracers to follow the fate of molecules within biological systems.
Geology and Geophysics:
Isotope geochemistry plays a crucial role in understanding Earth's history, the evolution of continents, and the formation of rocks and minerals. Radioactive isotopes are used for dating geological formations and understanding geochemical cycles.
Environmental Science:
Isotopes are used to trace pollutants in the environment, study water movement in aquifers, and monitor the effects of environmental changes. Isotopic analysis helps in understanding the sources and fate of contaminants.
Archaeology:
Radiocarbon dating, employing the radioactive isotope carbon-14, revolutionized archaeology by providing accurate dating of organic artifacts. This technique enables a more precise understanding of past civilizations and human history.
Conclusion: The Enduring Importance of Isotopes
Isotopes, though variations of the same element, hold profound significance across numerous scientific disciplines. Their differing physical properties and the unique characteristics of radioactive isotopes have led to transformative applications in medicine, industry, and scientific research. From medical imaging and cancer therapy to dating ancient artifacts and understanding Earth's history, the role of isotopes continues to expand, promising further groundbreaking advancements in the future. The ongoing research and development in isotope separation and analysis techniques will further unlock their potential, leading to even more impactful discoveries and applications. The study of isotopes remains a vibrant and essential field, driving innovation and progress across various sectors.
Latest Posts
Latest Posts
-
According To The Kinetic Theory Of Gases
Mar 17, 2025
-
Do Valence Electrons Have The Most Energy
Mar 17, 2025
-
Element Vs Compound Vs Homogeneous Vs Heterogeneous
Mar 17, 2025
-
For An Exothermic Reaction The Products
Mar 17, 2025
-
Fallacies Divide Into Roughly Two Kinds
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Atoms Of The Same Element With Varying Number Of Neutrons. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
