Describe The Periodic Trends For Atomic Radius
Muz Play
Mar 17, 2025 · 6 min read
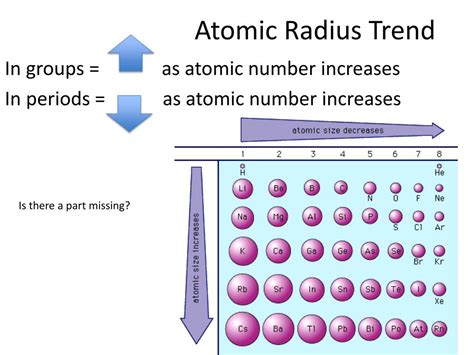
Table of Contents
Unveiling the Periodic Trends: A Deep Dive into Atomic Radius
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and recurring properties. Understanding these properties, particularly atomic radius, is crucial for predicting chemical behavior and reactivity. This comprehensive guide delves into the fascinating world of atomic radius, explaining its trends across the periodic table, the underlying reasons for these trends, and the subtle nuances that influence its variations.
What is Atomic Radius?
Atomic radius, simply put, is a measure of the size of an atom. However, defining a precise boundary for an atom is challenging because electrons don't orbit the nucleus in well-defined paths like planets around a star. Instead, they exist in a cloud of probability, described by orbitals. Therefore, there isn't a single definitive value for atomic radius. Instead, several methods are used to estimate it, leading to slightly varying results. Commonly used definitions include:
-
Covalent Radius: Half the distance between the nuclei of two identical atoms bonded together covalently. This is often used for nonmetals.
-
Metallic Radius: Half the distance between the nuclei of two adjacent atoms in a metallic crystal lattice. This applies to metals.
-
Van der Waals Radius: Half the distance between the nuclei of two identical, non-bonded atoms that are very close to each other. This method is relevant for noble gases and other weakly interacting atoms.
While different methods provide slightly different numerical values, the overall trends across the periodic table remain consistent regardless of the specific method used. For the purpose of this article, we will use the term "atomic radius" to refer to the general size of an atom, acknowledging the different methods used to determine it.
Periodic Trends in Atomic Radius: Across Periods and Down Groups
The periodic table reveals clear trends in atomic radius, influenced by two primary factors: effective nuclear charge and shielding effect.
Trend Across a Period (Left to Right):
Generally, atomic radius decreases as you move from left to right across a period. This is because:
-
Increased Effective Nuclear Charge: As you move across a period, the number of protons in the nucleus increases, increasing the positive charge. Simultaneously, electrons are added to the same principal energy level (shell). While the number of electrons increases, the added electrons are not very effective at shielding the outer electrons from the increased nuclear charge. This results in a stronger attraction between the nucleus and the outermost electrons, pulling them closer and reducing the atomic radius.
-
Shielding Effect Remains Relatively Constant: The shielding effect, provided by inner electrons reducing the attraction between the nucleus and outer electrons, remains relatively constant within a period. The added electrons are in the same energy level, and their shielding effect is not as significant as the increase in nuclear charge.
Example: Consider the period 2 elements: Lithium (Li) has a larger atomic radius than Neon (Ne). Ne has a much higher effective nuclear charge, pulling its outer electrons closer to the nucleus.
Trend Down a Group (Top to Bottom):
Atomic radius increases as you move down a group in the periodic table. This increase is due to:
-
Increased Principal Energy Levels: As you descend a group, electrons are added to successively higher principal energy levels (shells). Each shell is further away from the nucleus than the previous one. This increased distance significantly outweighs the increase in nuclear charge.
-
Increased Shielding Effect: The addition of inner shells of electrons significantly increases the shielding effect. The outer electrons are more effectively shielded from the nucleus's positive charge by the increased number of inner electrons. This reduces the effective nuclear charge experienced by the outer electrons.
Example: Consider Group 1 (alkali metals): Lithium (Li) has a smaller atomic radius than Cesium (Cs). Cs has significantly more electron shells, pushing its outer electrons farther from the nucleus.
Factors Affecting Atomic Radius: Beyond the Basics
While effective nuclear charge and shielding are the primary drivers of atomic radius trends, some subtle factors can influence the size of an atom:
Electron-Electron Repulsion:
The repulsion between electrons in the same energy level can slightly increase atomic radius. This effect is more pronounced in larger atoms with many electrons.
Nuclear Charge:
The magnitude of the positive charge in the nucleus directly affects the pull on the electrons. A greater nuclear charge results in a smaller atomic radius.
Penetration Effect:
Different subshells (s, p, d, f) within the same principal energy level have different penetration abilities toward the nucleus. 's' orbitals penetrate closer to the nucleus than 'p' orbitals, which penetrate closer than 'd' orbitals, and so on. This affects the shielding effect and, consequently, the atomic radius. Electrons in orbitals closer to the nucleus screen outer electrons more effectively.
Anomalous Behavior of Transition Metals:
Across the transition metal series (d-block elements), the atomic radius initially decreases slightly and then remains relatively constant or increases slightly. The reason for this less pronounced decrease is due to the effective shielding of the outer electrons by the inner 'd' electrons. The addition of electrons to the inner 'd' subshell doesn't significantly increase the effective nuclear charge acting on the outer 's' electrons.
Lanthanide Contraction:
This phenomenon is particularly important in understanding the size of the atoms in the later periods. The poor shielding effect of the 'f' electrons in the lanthanides leads to a greater-than-expected increase in effective nuclear charge, causing atoms in the subsequent periods (starting with the elements following the lanthanides) to be smaller than expected.
Isoelectronic Series:
Atoms or ions that have the same number of electrons are called isoelectronic. In an isoelectronic series, atomic radius decreases with an increase in nuclear charge. This is because a higher nuclear charge pulls the electrons more strongly towards the nucleus.
Applications of Understanding Atomic Radius
Understanding the trends in atomic radius is crucial in various fields of chemistry and related sciences:
-
Predicting Chemical Reactivity: Atomic radius plays a vital role in determining the reactivity of elements. Smaller atoms, with their greater nuclear charge, often have stronger attractions to electrons, influencing their ability to form bonds and participate in chemical reactions.
-
Ionic Radius and Crystal Structure: Atomic radius influences ionic radius, which is fundamental in understanding crystal structures and the properties of ionic compounds. The size of ions dictates the packing arrangement in crystal lattices.
-
Metallic Bonding and Properties: The atomic radius of metals plays a crucial role in determining the strength of metallic bonds and physical properties like melting point, density, and electrical conductivity.
-
Molecular Geometry and Properties: Atomic radius influences bond lengths and angles in molecules, affecting the overall shape and chemical properties of the molecule.
Conclusion: A Complex but Essential Concept
While a simple concept at first glance, atomic radius reveals a rich tapestry of interactions within the atom and is critical for understanding the behaviour of elements. The interplay of effective nuclear charge, shielding effect, electron-electron repulsion, and other subtle factors, creates a complex landscape of atomic sizes that profoundly impacts chemical and physical properties. By grasping the periodic trends and influencing factors, we gain valuable insights into the world of chemical reactivity, bonding, and materials science. The atomic radius is not merely a number; it's a key to unlocking a deeper understanding of the periodic table and the elements that make up our universe.
Latest Posts
Latest Posts
-
Strong And Weak Acids And Bases List
Mar 17, 2025
-
Absorption Spectrum Of Helium Largest Transition
Mar 17, 2025
-
List Of Equipments For Validation In Pharma And Biotech Industry
Mar 17, 2025
-
An Example Of Extensive Property Of Matter Is
Mar 17, 2025
-
What Is The Amount Of Matter In An Object
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Describe The Periodic Trends For Atomic Radius . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
