Difference Between Compound And Homogeneous Mixture
Muz Play
Mar 25, 2025 · 6 min read
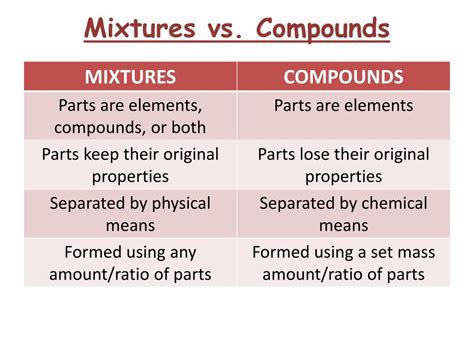
Table of Contents
Delving Deep: The Crucial Differences Between Compound and Homogeneous Mixtures
Understanding the fundamental differences between compounds and homogeneous mixtures is crucial for grasping the basics of chemistry. While both involve combining different substances, the nature of their bonding and the resulting properties differ significantly. This article will explore these differences in detail, providing a comprehensive understanding of each type of mixture and clarifying the key distinctions. We'll delve into their definitions, characteristics, properties, examples, and separation methods, equipping you with a solid foundation in chemistry.
What is a Compound?
A compound is a pure substance formed when two or more different chemical elements are chemically bonded together. This chemical bonding involves the sharing or transfer of electrons between atoms, resulting in a new substance with entirely different properties than the original elements. The key characteristic is the fixed ratio in which the elements combine. This fixed ratio is defined by the chemical formula of the compound. For instance, water (H₂O) always has two hydrogen atoms for every one oxygen atom. You cannot change this ratio and still have water. Change the ratio, and you get a completely different substance.
Key Characteristics of Compounds:
- Chemically Bonded: Atoms in a compound are held together by strong chemical bonds (ionic, covalent, or metallic). These bonds are significantly stronger than the intermolecular forces holding together components of mixtures.
- Fixed Composition: The elements in a compound are always present in a definite, fixed proportion by mass. This proportion is represented by its chemical formula.
- New Properties: Compounds exhibit properties that are significantly different from the elements that compose them. For example, sodium (a highly reactive metal) and chlorine (a toxic gas) combine to form sodium chloride (table salt), a harmless, crystalline solid.
- Chemical Formulas: Compounds are represented by chemical formulas, which indicate the types and number of atoms of each element present in the compound.
- Decomposition: Compounds can be broken down into their constituent elements through chemical reactions. This requires energy input, usually in the form of heat or electricity.
Examples of Compounds:
- Water (H₂O): Formed from hydrogen and oxygen.
- Carbon Dioxide (CO₂): Formed from carbon and oxygen.
- Sodium Chloride (NaCl): Formed from sodium and chlorine.
- Glucose (C₆H₁₂O₆): Formed from carbon, hydrogen, and oxygen.
- Sulfuric Acid (H₂SO₄): Formed from hydrogen, sulfur, and oxygen.
What is a Homogeneous Mixture?
A homogeneous mixture, also known as a solution, is a mixture where the components are uniformly distributed throughout the mixture. At a macroscopic level (visible to the naked eye), the mixture appears to be completely uniform in composition. This means that no matter what sample you take from the mixture, its composition will be the same. Crucially, the components are not chemically bonded.
Key Characteristics of Homogeneous Mixtures:
- Uniform Composition: The components are evenly distributed throughout the mixture.
- Not Chemically Bonded: The components retain their individual chemical identities and properties. They are physically mixed, not chemically combined.
- Single Phase: A homogeneous mixture exists in a single phase (solid, liquid, or gas). You won't see distinct layers or regions of different composition.
- Separation: The components of a homogeneous mixture can be separated using physical methods like distillation, evaporation, filtration, or chromatography.
Examples of Homogeneous Mixtures:
- Saltwater: Salt (NaCl) is dissolved uniformly in water (H₂O).
- Air: A mixture of various gases, including nitrogen, oxygen, argon, and carbon dioxide, uniformly distributed.
- Sugar dissolved in water: Sugar molecules are dispersed evenly throughout the water.
- Brass: An alloy of copper and zinc, with a uniform composition throughout.
- Steel: An alloy of iron and carbon, exhibiting a consistent composition.
The Crucial Differences: A Comparative Analysis
The table below summarizes the key differences between compounds and homogeneous mixtures:
| Feature | Compound | Homogeneous Mixture |
|---|---|---|
| Bonding | Chemically bonded | Not chemically bonded |
| Composition | Fixed, definite proportion by mass | Variable, components can vary in proportion |
| Properties | Properties different from constituent elements | Properties similar to constituent components |
| Separation | Requires chemical reaction | Separable by physical methods |
| Formula | Has a chemical formula | Does not have a chemical formula |
| Phase | Can exist in various phases | Generally exists in a single phase |
| Example | Water (H₂O), Sodium Chloride (NaCl) | Saltwater, Air, Brass |
Methods for Separating Components
The methods used to separate the components of a compound and a homogeneous mixture are vastly different, reflecting the fundamental nature of their composition:
Separating Compounds:
Separating the elements of a compound requires breaking the chemical bonds that hold them together. This is accomplished through chemical reactions, which often require significant energy input, such as:
- Electrolysis: Using an electric current to break down a compound, commonly used for separating water into hydrogen and oxygen.
- Thermal Decomposition: Applying heat to decompose a compound, often employed in the extraction of certain metals from their ores.
- Chemical Reactions: Using a chemical reagent to react with the compound and break it down into its constituent elements or simpler compounds.
Separating Homogeneous Mixtures:
Separating the components of a homogeneous mixture only requires utilizing physical methods that exploit differences in their physical properties. These methods include:
- Distillation: Separating liquids with different boiling points by heating the mixture and condensing the vapor. This is commonly used to separate water from dissolved salt.
- Evaporation: Removing a solvent (usually water) by heating the mixture, leaving behind the dissolved solute. This is useful for separating salt from saltwater.
- Filtration: Separating solids from liquids using a porous material that allows the liquid to pass through but retains the solid.
- Chromatography: Separating components based on their differing affinities for a stationary and mobile phase. This technique is used extensively in analytical chemistry.
- Crystallization: Separating a solid solute from a liquid solution by allowing the solution to cool slowly, forming crystals of the solute.
Heterogeneous Mixtures: A Brief Comparison
While this article focuses on the difference between compounds and homogeneous mixtures, it's worth briefly mentioning heterogeneous mixtures. Unlike homogeneous mixtures, heterogeneous mixtures have a non-uniform composition. You can visually distinguish different components or phases within the mixture. Examples include sand and water, oil and water, and a salad. The components of heterogeneous mixtures are generally easier to separate than those of homogeneous mixtures, often using simple physical methods like decantation or filtration.
Conclusion
The distinction between compounds and homogeneous mixtures is a cornerstone of chemistry. Compounds are formed by the chemical bonding of elements, resulting in substances with entirely new properties, while homogeneous mixtures are physical combinations where components retain their individual identities and are uniformly distributed. Understanding these differences is essential for comprehending the behavior and properties of matter. This knowledge is crucial for various fields, from material science and engineering to medicine and environmental science. By mastering this fundamental concept, you lay a solid groundwork for further exploration in the fascinating world of chemistry.
Latest Posts
Latest Posts
-
Using Hesss Law To Calculate Net Reaction Enthalpy
Mar 26, 2025
-
Solids Have A Definite Shape Because
Mar 26, 2025
-
What Is A Subscript In A Chemical Equation
Mar 26, 2025
-
How To Do Post Closing Trial Balance
Mar 26, 2025
-
Como Multiplicar Dos Raices Cuadradas Dividas Entre Otra Raiz Cuadrada
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Difference Between Compound And Homogeneous Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
