Difference Between Ionic And Molecular Compound
Muz Play
Mar 20, 2025 · 6 min read
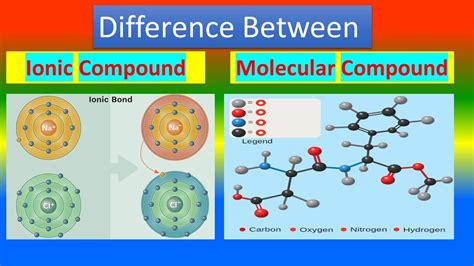
Table of Contents
Delving Deep into the Differences: Ionic vs. Molecular Compounds
Understanding the fundamental differences between ionic and molecular compounds is crucial for anyone studying chemistry. These differences extend beyond simple definitions; they dictate the physical and chemical properties of substances, influencing their applications in various fields. This comprehensive guide will explore the core distinctions between these two major classes of compounds, covering their formation, properties, and practical examples.
Formation: A Tale of Two Bonds
The primary difference between ionic and molecular compounds lies in the type of chemical bond that holds their constituent atoms together. This, in turn, dictates their structure and properties.
Ionic Compounds: The Electrostatic Attraction
Ionic compounds are formed through the electrostatic attraction between positively charged ions (cations) and negatively charged ions (anions). This occurs when atoms with significantly different electronegativities interact. Electronegativity refers to an atom's ability to attract electrons within a chemical bond. A large electronegativity difference leads to one atom effectively transferring an electron (or electrons) to another. The atom that loses electrons becomes a positively charged cation, while the atom that gains electrons becomes a negatively charged anion.
The strong Coulombic forces between these oppositely charged ions create a strong ionic bond, resulting in a crystal lattice structure. This lattice is a repeating three-dimensional arrangement of ions, maximizing electrostatic attraction and minimizing repulsion. The strength of this electrostatic attraction is directly related to the charge of the ions and the distance between them. Higher charges and shorter distances lead to stronger ionic bonds.
Examples of Ionic Compound Formation:
- Sodium Chloride (NaCl): Sodium (Na), a metal with low electronegativity, readily loses one electron to become a Na⁺ cation. Chlorine (Cl), a nonmetal with high electronegativity, readily gains one electron to become a Cl⁻ anion. The electrostatic attraction between Na⁺ and Cl⁻ forms NaCl, common table salt.
- Magnesium Oxide (MgO): Magnesium (Mg) loses two electrons to form Mg²⁺, while oxygen (O) gains two electrons to form O²⁻. The resulting strong ionic bond forms MgO.
- Calcium Chloride (CaCl₂): Calcium (Ca) loses two electrons to form Ca²⁺, and each chlorine atom gains one electron to form Cl⁻. Two chloride ions are needed to balance the 2+ charge of the calcium ion.
Molecular Compounds: The Sharing is Caring Approach
Molecular compounds, on the other hand, are formed through the sharing of electrons between atoms. This sharing creates a covalent bond, where atoms achieve a stable electron configuration by sharing electrons to complete their valence shells. This typically occurs between atoms with similar or relatively small electronegativity differences. Instead of a complete transfer of electrons, atoms essentially "pool" their electrons to create a shared electron cloud.
Unlike ionic compounds, molecular compounds do not form a continuous lattice structure. Instead, they exist as discrete molecules – groups of atoms bonded together – with relatively weak intermolecular forces holding the molecules together.
Examples of Molecular Compound Formation:
- Water (H₂O): Each hydrogen atom shares one electron with the oxygen atom, forming two covalent bonds. Oxygen shares two of its electrons, one with each hydrogen atom.
- Carbon Dioxide (CO₂): Carbon shares two electrons with each of the two oxygen atoms, forming two double covalent bonds.
- Methane (CH₄): Carbon shares one electron with each of the four hydrogen atoms, forming four single covalent bonds.
Properties: A Clear Distinction
The fundamental differences in bonding lead to significant variations in the physical and chemical properties of ionic and molecular compounds.
Physical Properties: A Contrast in Behavior
| Property | Ionic Compounds | Molecular Compounds |
|---|---|---|
| Melting Point | High (strong electrostatic forces) | Low (weak intermolecular forces) |
| Boiling Point | High (strong electrostatic forces) | Low (weak intermolecular forces) |
| Solubility | Often soluble in polar solvents (water) | Solubility varies greatly depending on polarity |
| Conductivity | Conducts electricity when molten or dissolved | Generally does not conduct electricity |
| Hardness | Usually hard and brittle | Generally soft |
| State at Room Temperature | Often solid | Can be solid, liquid, or gas |
Chemical Properties: Reactivity and Reactions
Ionic compounds tend to be more reactive than molecular compounds due to the relatively weaker electrostatic interactions holding the individual ions together. This also affects their behavior in aqueous solutions. Molecular compounds' reactivity is largely determined by the types of atoms and bonds present within the molecule.
- Ionic compounds readily dissociate in polar solvents: This leads to the formation of ions in solution, which can readily participate in chemical reactions.
- Molecular compounds typically require higher activation energy for reactions: The covalent bonds within the molecule must be broken before reaction can occur.
Examples in Everyday Life: The Ubiquity of Both
Both ionic and molecular compounds are ubiquitous in our daily lives, playing crucial roles in various applications.
Ionic Compounds in Action
- Table Salt (NaCl): Essential for human health, used in food preservation, and in numerous industrial processes.
- Calcium Carbonate (CaCO₃): A major component of limestone, marble, and chalk, used in construction and as a dietary supplement.
- Sodium Hydroxide (NaOH): A strong base used in various industrial applications, including soap making and paper production.
- Potassium Chloride (KCl): Used as a fertilizer and in medical applications.
Molecular Compounds Around Us
- Water (H₂O): Essential for life, used as a solvent, and in various industrial processes.
- Carbon Dioxide (CO₂): A greenhouse gas essential for photosynthesis, used in carbonated beverages and fire extinguishers.
- Glucose (C₆H₁₂O₆): A simple sugar, a primary source of energy for living organisms.
- Ethanol (C₂H₅OH): Used as a solvent, fuel, and in alcoholic beverages.
- Polymers (e.g., polyethylene, nylon): Large molecules with repeating units, used in plastics, fabrics, and numerous other applications.
Identifying the Compound Type: Practical Tips
Distinguishing between ionic and molecular compounds can be straightforward with a little understanding of their properties and the periodic table.
- Examine the elements involved: Ionic compounds typically involve a metal and a nonmetal. Molecular compounds are usually composed of nonmetals only. However, there are exceptions.
- Consider the electronegativity difference: A large electronegativity difference suggests an ionic bond. A small difference suggests a covalent bond.
- Look at the physical properties: High melting and boiling points, hardness, and conductivity in solution often indicate an ionic compound. Low melting and boiling points and lack of conductivity usually indicate a molecular compound.
Conclusion: A Fundamental Distinction with Broad Implications
The distinction between ionic and molecular compounds is fundamental to chemistry. Understanding the differences in their formation, properties, and applications is crucial for grasping the behavior of matter and its diverse applications in various fields, from medicine and materials science to environmental studies and beyond. By understanding these core differences, you gain a powerful tool for interpreting the behavior and characteristics of numerous substances that make up our world.
Latest Posts
Latest Posts
-
This Type Of Reaction Is Spontaneous And Releases Energy
Mar 20, 2025
-
The Vertical Column Of The Periodic Table
Mar 20, 2025
-
A Box Is Given A Sudden Push Up A Ramp
Mar 20, 2025
-
How To Read H Nmr Spectrum
Mar 20, 2025
-
Is Displacement A Scalar Or Vector
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Difference Between Ionic And Molecular Compound . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
