Differentiate Between Empirical Formula And Molecular Formula
Muz Play
Mar 22, 2025 · 6 min read
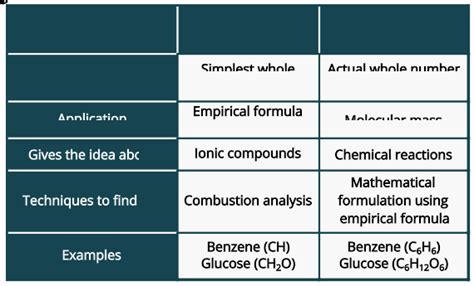
Table of Contents
Differentiating Between Empirical Formula and Molecular Formula: A Comprehensive Guide
Understanding the fundamental differences between empirical and molecular formulas is crucial for anyone studying chemistry. While both represent the composition of a compound, they provide different levels of information. This comprehensive guide will delve deep into the distinctions, exploring their definitions, how to determine them, and practical examples to solidify your understanding. We'll also touch upon the applications of both formulas in various chemical contexts.
What is an Empirical Formula?
The empirical formula of a compound represents the simplest whole-number ratio of atoms of each element present in the compound. It doesn't necessarily reflect the actual number of atoms in a molecule; it simply shows the ratio. Think of it as the most reduced form of a chemical formula. For instance, the empirical formula doesn't tell you how many atoms are actually in a molecule, just the ratio between them.
Key characteristics of an empirical formula:
- Simplest Ratio: Always expresses the ratio of atoms in the simplest whole-number form.
- Doesn't show the actual number of atoms: It only indicates the relative proportion of elements.
- Can be the same as the molecular formula: If the molecular formula already represents the simplest ratio, the empirical and molecular formulas are identical.
- Determined from experimental data: The empirical formula is derived from experimental analysis, typically through techniques like combustion analysis.
How to Determine an Empirical Formula
Determining the empirical formula involves several steps:
-
Find the mass of each element: This information is usually provided in the problem or obtained from experimental data (e.g., percentage composition).
-
Convert mass to moles: Divide the mass of each element by its molar mass (atomic weight found on the periodic table). This gives the number of moles of each element.
-
Find the mole ratio: Divide the number of moles of each element by the smallest number of moles calculated in the previous step. This gives the simplest whole-number ratio of atoms.
-
Express as a formula: Write the elements as subscripts corresponding to the mole ratios obtained.
Example:
Let's say a compound is found to contain 75% carbon and 25% hydrogen by mass.
-
Mass: Assume a 100g sample. This gives 75g Carbon and 25g Hydrogen.
-
Moles:
- Moles of Carbon = 75g / 12.01 g/mol (molar mass of C) ≈ 6.24 moles
- Moles of Hydrogen = 25g / 1.01 g/mol (molar mass of H) ≈ 24.75 moles
-
Mole Ratio:
- Carbon: 6.24 moles / 6.24 moles ≈ 1
- Hydrogen: 24.75 moles / 6.24 moles ≈ 4
-
Empirical Formula: CH₄ (Methane)
What is a Molecular Formula?
The molecular formula represents the actual number of atoms of each element present in a molecule of a compound. It provides a complete picture of the composition of a single molecule. It's the true reflection of the atomic composition of a compound.
Key characteristics of a molecular formula:
- Actual Number of Atoms: Shows the exact number of atoms of each element in one molecule.
- Multiple of the Empirical Formula: The molecular formula is always a whole-number multiple of the empirical formula.
- Provides more information than the empirical formula: It gives a complete representation of the molecule's composition.
- Requires additional information: Determining the molecular formula requires knowledge of the compound's molar mass (molecular weight) in addition to the empirical formula.
How to Determine a Molecular Formula
Determining the molecular formula requires the empirical formula and the molar mass of the compound.
-
Calculate the empirical formula mass: Add up the molar masses of all atoms in the empirical formula.
-
Find the whole-number multiple: Divide the molar mass of the compound by the empirical formula mass. This gives the whole number by which the subscripts in the empirical formula must be multiplied.
-
Write the molecular formula: Multiply the subscripts in the empirical formula by the whole number obtained in the previous step.
Example:
Let's say we have the empirical formula CH₂O (which is the empirical formula for many simple sugars). Suppose the molar mass of the compound is experimentally determined to be 180.16 g/mol.
-
Empirical Formula Mass: 12.01 g/mol (C) + 2 * 1.01 g/mol (H) + 16.00 g/mol (O) = 30.03 g/mol
-
Whole-Number Multiple: 180.16 g/mol / 30.03 g/mol ≈ 6
-
Molecular Formula: (CH₂O)₆ = C₆H₁₂O₆ (Glucose)
Key Differences Summarized
| Feature | Empirical Formula | Molecular Formula |
|---|---|---|
| Represents | Simplest whole-number ratio of atoms | Actual number of atoms in a molecule |
| Information | Relative proportions of elements | Exact composition of a molecule |
| Determination | From elemental analysis (mass or percentage) | Requires empirical formula and molar mass |
| Relationship | Always a simpler form of the molecular formula | A whole-number multiple of the empirical formula |
Applications of Empirical and Molecular Formulas
Both empirical and molecular formulas play vital roles in various chemical applications:
-
Chemical Analysis: Empirical formulas are fundamental in identifying unknown compounds based on their elemental composition. This is commonly used in forensic science, environmental monitoring, and materials science.
-
Stoichiometry: Both formulas are crucial in stoichiometric calculations, allowing chemists to determine the amounts of reactants and products involved in chemical reactions. Molecular formulas are especially essential for accurate calculations involving molar masses.
-
Organic Chemistry: The determination of both empirical and molecular formulas is crucial for characterizing organic compounds, understanding their structures, and predicting their properties.
-
Polymer Chemistry: Empirical formulas are commonly used to represent the repeating units in polymers, while the molecular formula often depicts the overall composition of a polymer chain (although the chain length can be variable).
-
Biochemistry: The molecular formulas of biological molecules like proteins, carbohydrates, and nucleic acids are essential for understanding their structures, functions, and interactions within living organisms.
Beyond the Formulas: Understanding Chemical Structure
While empirical and molecular formulas provide essential information about the composition of compounds, they don't fully reveal the arrangement of atoms within a molecule. This structural information is crucial for understanding a molecule's properties and reactivity. Techniques like spectroscopy (NMR, IR, Mass Spec) and X-ray crystallography are employed to determine the detailed structure of molecules.
Conclusion
Understanding the distinction between empirical and molecular formulas is a cornerstone of chemical understanding. While the empirical formula provides a simplified representation of the ratio of elements, the molecular formula reveals the precise composition of a molecule. Knowing how to determine both, and understanding their limitations, is key to making accurate predictions and solving a wide range of chemical problems. Remember that the molecular formula builds upon the foundation of the empirical formula and provides a more complete picture of a compound's chemical identity. Mastering these concepts is essential for success in any chemistry-related field.
Latest Posts
Latest Posts
-
Is Copper A Metal Nonmetal Or Metalloid
Mar 22, 2025
-
American History Reconstruction To The Present
Mar 22, 2025
-
Valence Bond Theory Vs Molecular Orbital Theory
Mar 22, 2025
-
How To Find Ph At The Equivalence Point
Mar 22, 2025
-
Does He Have The Same Line Emission Spectrum As H
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Differentiate Between Empirical Formula And Molecular Formula . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
