Does Liquid Have A Definite Shape And Volume
Muz Play
Mar 30, 2025 · 5 min read
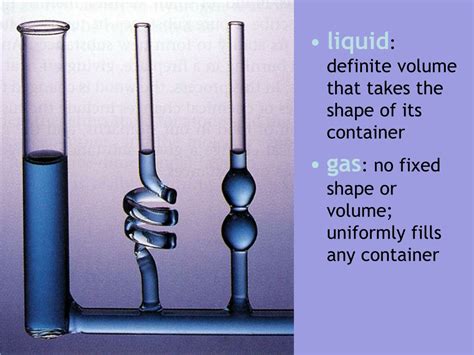
Table of Contents
Does Liquid Have a Definite Shape and Volume? Exploring the Properties of Matter
The question of whether liquids possess a definite shape and volume is fundamental to understanding the states of matter. While seemingly simple, the answer reveals much about the intricate behavior of molecules and their interactions. This article will delve deep into the properties of liquids, comparing and contrasting them with solids and gases, and providing a clear understanding of their shape and volume characteristics. We'll also explore the scientific principles underlying these properties.
Understanding States of Matter: Solid, Liquid, and Gas
Before we specifically address liquids, let's establish a basic understanding of the three primary states of matter: solid, liquid, and gas. These states are differentiated primarily by the arrangement and movement of their constituent particles (atoms or molecules).
Solids: Definite Shape and Volume
Solids are characterized by their definite shape and volume. Their particles are tightly packed together in a highly ordered arrangement, held in place by strong intermolecular forces. This rigid structure prevents the solid from changing its shape or volume easily. Think of a block of ice; it maintains its shape and size regardless of the container it's in. Applying force might cause deformation, but the solid will generally resist significant changes in shape or volume.
Liquids: Definite Volume, Indefinite Shape
Liquids, unlike solids, have a definite volume but an indefinite shape. Their particles are still relatively close together, but they possess more kinetic energy, allowing them to move past one another. This mobility enables liquids to flow and take the shape of their container. The volume, however, remains constant unless significantly compressed. Imagine pouring water into a glass; the water adapts to the shape of the glass but maintains the same overall volume.
Gases: Indefinite Shape and Volume
Gases possess neither a definite shape nor a definite volume. Their particles are widely dispersed and move rapidly and randomly. They expand to fill the available space, readily changing both their shape and volume depending on the container they occupy. Think of air in a balloon; the air conforms to the balloon's shape, and if the balloon is expanded, the air also expands to fill the larger volume.
The Molecular Dance: Understanding Liquid Behavior
The behavior of liquids, specifically their indefinite shape and definite volume, is directly related to the nature of intermolecular forces and the kinetic energy of their molecules.
Intermolecular Forces: The Glue That Holds (Sort Of)
Intermolecular forces are the attractive forces between molecules. These forces are weaker than the intramolecular forces (bonds within a molecule) but are crucial in determining the state of matter. In liquids, these forces are strong enough to hold the molecules relatively close together, maintaining a definite volume. However, they are not strong enough to restrict the movement of molecules, allowing them to slide past each other and adopt the shape of their container.
Kinetic Energy: The Movers and Shakers
The kinetic energy of molecules determines their movement. In liquids, molecules possess enough kinetic energy to overcome some of the intermolecular forces, resulting in their fluidity. This allows them to flow and conform to the shape of the container, while still maintaining a constant volume due to the balance between intermolecular attraction and kinetic energy.
The In-Between State: Bridging Solid and Gas
Liquids occupy a unique position between solids and gases. They possess some of the characteristics of solids (definite volume) and some of the characteristics of gases (indefinite shape). This intermediary state is a consequence of the balance between intermolecular forces and kinetic energy.
Factors Affecting Liquid Shape and Volume
Several factors influence the behavior of liquids, affecting their apparent shape and volume:
Temperature: A Major Influencer
Temperature significantly impacts the kinetic energy of molecules. Increasing temperature increases kinetic energy, causing molecules to move more rapidly and overcome intermolecular forces more effectively. This leads to a slight increase in volume (thermal expansion) and a decrease in viscosity (resistance to flow). Conversely, decreasing temperature reduces kinetic energy, slowing molecular movement and increasing the effect of intermolecular forces.
Pressure: Compressing Liquids
While liquids are relatively incompressible compared to gases, they are still slightly affected by pressure. Increasing pressure forces the molecules closer together, resulting in a slight decrease in volume. However, this change in volume is typically insignificant compared to the change experienced by gases under similar pressure changes.
Surface Tension: The Skin Effect
Surface tension is a property of liquids where molecules at the surface experience a net inward force, creating a "skin-like" effect. This force minimizes the surface area of the liquid, influencing the shape of the liquid, particularly in small quantities or when interacting with other surfaces.
Viscosity: The Resistance to Flow
Viscosity refers to a liquid's resistance to flow. High-viscosity liquids, such as honey, flow more slowly than low-viscosity liquids, such as water. Viscosity is influenced by intermolecular forces, temperature, and the shape of molecules.
Beyond the Basics: Advanced Concepts
The simple explanation of liquids having a definite volume and indefinite shape requires further exploration for a comprehensive understanding.
Compressibility: A Closer Look
While often described as incompressible, liquids are compressible, albeit to a much lesser extent than gases. The degree of compressibility depends on the intermolecular forces and the temperature. High pressure can lead to noticeable volume changes, particularly in specialized applications.
Density and Buoyancy: The Weight of Liquids
The density of a liquid plays a crucial role in its behavior. Density refers to the mass per unit volume of the liquid. Differences in density drive buoyancy – the upward force exerted on an object immersed in a liquid. This principle is fundamental to understanding phenomena like floating and sinking.
Conclusion: Liquids – A Dynamic State of Matter
Liquids represent a fascinating state of matter, characterized by a definite volume and an indefinite shape. This behavior is a direct consequence of the intricate interplay between intermolecular forces and the kinetic energy of their molecules. Understanding these properties is critical in diverse fields, from engineering and chemistry to meteorology and biology. The seemingly straightforward concept of liquid shape and volume leads us to a deeper appreciation of the complex and dynamic nature of the physical world. This nuanced understanding, incorporating factors like temperature, pressure, surface tension, and viscosity, allows for accurate prediction and manipulation of liquid behavior in various applications.
Latest Posts
Latest Posts
-
Moment Of Inertia Of A Point
Apr 01, 2025
-
Cross Section Of A Woody Stem
Apr 01, 2025
-
A State Function Is Best Described As
Apr 01, 2025
-
What Is The Electron Configuration For Ne
Apr 01, 2025
-
Difference Between E1 And E2 Reaction
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Does Liquid Have A Definite Shape And Volume . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
