Does Polymerization Of Styrene Create A Simple Backbone
Muz Play
Mar 16, 2025 · 5 min read
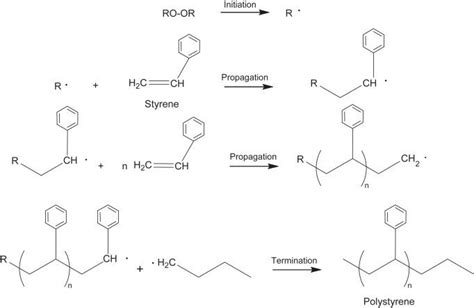
Table of Contents
Does Polymerization of Styrene Create a Simple Backbone? Exploring the Structure and Properties of Polystyrene
The polymerization of styrene, a simple aromatic vinyl monomer, leads to the formation of polystyrene (PS), a widely used polymer with a seemingly simple backbone. However, a closer examination reveals a more nuanced picture of its structure and properties, which are far from simplistic. This article delves deep into the intricacies of polystyrene's backbone, exploring its structural characteristics, influencing factors during polymerization, and the consequential impact on its macroscopic properties.
Understanding the Styrene Monomer and its Polymerization
Styrene, chemically known as vinylbenzene (C₈H₈), possesses a vinyl group (-CH=CH₂) directly attached to a phenyl ring. This combination of a reactive vinyl group and a bulky, aromatic phenyl ring dictates the characteristics of the resulting polymer. The polymerization process, typically initiated by free radical, anionic, or cationic methods, involves the sequential addition of styrene monomers. Each monomer adds to the growing polymer chain via the double bond's opening, creating a carbon-carbon bond.
The Basic Backbone: A Chain of Carbon Atoms
The simplest representation of polystyrene's backbone shows a linear chain of carbon atoms. Each carbon atom, originally part of the vinyl group in styrene, forms single bonds with two other carbons in the chain and a phenyl ring. This seemingly straightforward arrangement gives rise to the polymer's basic structural unit:
-CH₂-CH(C₆H₅)-CH₂-CH(C₆H₅)-CH₂-CH(C₆H₅)-
This repeated unit, representing the backbone's fundamental structure, is crucial in understanding polystyrene's properties. However, the reality is far more complex.
Beyond the Simple Backbone: Factors Influencing Polystyrene Structure
The "simple" backbone description is an oversimplification. Several factors influence the actual structure of the polystyrene chain:
1. Tacticity: The Arrangement of Phenyl Groups
The arrangement of the phenyl groups along the polymer backbone significantly impacts its properties. Polystyrene can exist in three main tactic forms:
-
Isotactic polystyrene: All phenyl groups are on the same side of the polymer chain. This results in a highly ordered structure with increased crystallinity and higher melting point.
-
Syndiotactic polystyrene: Phenyl groups alternate sides along the chain. This configuration also leads to a relatively ordered structure, though less so than isotactic polystyrene.
-
Atactic polystyrene: Phenyl groups are randomly arranged along the chain. This is the most common type of polystyrene produced commercially, characterized by an amorphous structure and lower melting point.
The tacticity is primarily determined by the polymerization method and conditions. For example, anionic polymerization under controlled conditions can lead to high isotacticity or syndiotacticity. However, the free-radical polymerization method, commonly used for commercial production, produces primarily atactic polystyrene.
2. Branching and Molecular Weight Distribution: Impact on Physical Properties
The polymerization process may also lead to chain branching, where side chains sprout from the main backbone. Branching alters the polymer's morphology, influencing its flow behavior and mechanical strength. Moreover, the molecular weight distribution (MWD) of the polymer plays a vital role. A broad MWD indicates a mixture of chains with varying lengths, leading to a range of physical properties. A narrow MWD, on the other hand, provides more consistent and predictable behavior.
3. Steric Hindrance: The Role of the Phenyl Ring
The bulky phenyl ring attached to the backbone introduces steric hindrance, affecting the chain conformation and flexibility. The presence of the phenyl ring restricts rotation around the carbon-carbon bonds of the backbone, influencing the polymer's rigidity and glass transition temperature (Tg). The steric interaction between phenyl rings on neighboring chains also impacts intermolecular forces, contributing to the material's mechanical properties.
4. Chain Conformation: Coils, Helices, and Random Walks
The polystyrene chain does not exist as a perfectly straight line. Due to rotational flexibility around the bonds and steric hindrance from the phenyl groups, the chains adopt various conformations, including random coils, helices, and other more complex arrangements. This conformational variability plays a crucial role in determining the polymer's bulk properties, including its solubility, viscosity, and mechanical strength.
The Macroscopic Properties: A Consequence of the Backbone Structure
The intricate details of the polystyrene backbone structure directly influence its macroscopic properties. These properties dictate its numerous applications:
1. Amorphous Nature and Glass Transition Temperature
Atactic polystyrene, the dominant commercial form, is amorphous, meaning its chains lack long-range order. The glass transition temperature (Tg) of atactic polystyrene is around 100°C. Below this temperature, the polymer is glassy and brittle; above Tg, it becomes rubbery and more flexible. This Tg value is significantly influenced by the tacticity and molecular weight of the polymer.
2. Mechanical Properties: Strength, Rigidity, and Impact Resistance
The backbone structure dictates polystyrene's mechanical properties. Atactic polystyrene is relatively brittle with moderate tensile strength and stiffness. Its impact resistance is limited due to the amorphous nature and lack of strong intermolecular forces. Modifications like copolymerization or blending with other polymers can improve these properties.
3. Optical Properties: Transparency and Clarity
Polystyrene is transparent and exhibits good optical clarity, making it suitable for applications requiring light transmission. This optical clarity arises from the relatively regular arrangement of the chains at a microscopic level and the absence of strong light scattering centers.
4. Thermal Properties: Melting Point and Degradation
Atactic polystyrene does not have a sharp melting point due to its amorphous nature. However, it undergoes thermal degradation at temperatures above 300°C. Isotactic and syndiotactic polystyrene exhibit higher melting points because of their higher degree of crystallinity.
Conclusion: A Simple Backbone with Complex Consequences
While the basic backbone of polystyrene can be simply described as a linear chain of carbon atoms, the reality is far more intricate. Factors like tacticity, branching, molecular weight distribution, steric hindrance, and chain conformation significantly impact the polymer's structure and resulting properties. Understanding these nuances is crucial for tailoring polystyrene's characteristics to specific applications. The seemingly "simple" backbone of polystyrene gives rise to a material with diverse properties, making it a versatile polymer in various industries. Further research into controlling the polymerization process and understanding the relationships between molecular structure and macroscopic properties will continue to expand the applications of this widely used material. Therefore, while the basic repeat unit is simple, the implications for its physical properties are far from straightforward.
Latest Posts
Latest Posts
-
According To The Kinetic Theory Of Gases
Mar 17, 2025
-
Do Valence Electrons Have The Most Energy
Mar 17, 2025
-
Element Vs Compound Vs Homogeneous Vs Heterogeneous
Mar 17, 2025
-
For An Exothermic Reaction The Products
Mar 17, 2025
-
Fallacies Divide Into Roughly Two Kinds
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Does Polymerization Of Styrene Create A Simple Backbone . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
