Examples Of A Liquid Dissolved In A Liquid
Muz Play
Mar 18, 2025 · 5 min read
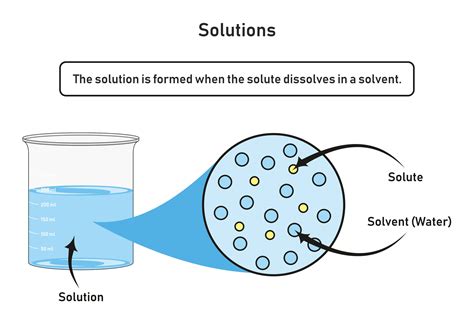
Table of Contents
Examples of a Liquid Dissolved in a Liquid: A Deep Dive into Solutions
Solutions are ubiquitous in our daily lives, from the beverages we drink to the cleaning products we use. A solution, in its simplest form, is a homogeneous mixture of two or more substances. This article focuses specifically on liquid-liquid solutions, where one liquid is dissolved in another. Understanding these solutions is crucial across various scientific fields, from chemistry and biology to engineering and environmental science. We’ll explore numerous examples, delve into the underlying principles governing their formation, and touch upon their applications.
Understanding Liquid-Liquid Solutions
Before we explore specific examples, let’s establish a fundamental understanding. In a liquid-liquid solution, the substance present in the larger amount is called the solvent, while the substance dissolved in the solvent is called the solute. The solute particles are distributed evenly throughout the solvent at the molecular level, resulting in a homogenous mixture. The ability of one liquid to dissolve in another depends on several factors, including:
-
Polarity: "Like dissolves like" is a fundamental principle. Polar solvents tend to dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes. Polarity refers to the distribution of electrical charge within a molecule. Water, for example, is a highly polar solvent.
-
Intermolecular forces: The strength of the attractive forces between molecules (e.g., hydrogen bonding, dipole-dipole interactions, London dispersion forces) plays a significant role in solubility. Stronger interactions between solute and solvent molecules favor dissolution.
-
Temperature: Temperature often influences solubility. Increasing temperature generally increases the solubility of solids and liquids in liquids, though there are exceptions.
-
Pressure: Pressure typically has a minimal effect on the solubility of liquids in liquids compared to the effect it has on gases in liquids.
Diverse Examples of Liquid-Liquid Solutions
Now, let's delve into a wide array of examples, categorizing them for better understanding.
Everyday Examples:
-
Alcoholic Beverages: The quintessential example! Ethanol (alcohol) is dissolved in water in various alcoholic beverages like beer, wine, and spirits. The concentration of ethanol varies significantly across these drinks.
-
Vinegar: Vinegar is an aqueous solution of acetic acid, a weak organic acid. The percentage of acetic acid determines the strength of the vinegar.
-
Fruit Juices: Many fruit juices are liquid-liquid solutions. Water is the solvent, and various sugars, acids (like citric acid), and other flavor compounds act as solutes.
-
Soft Drinks: Carbonated soft drinks are a complex example. Water is the primary solvent, dissolving sugars, flavorings, and carbon dioxide (a gas, but in solution). The dissolved carbon dioxide contributes to the fizzy nature of the drink.
-
Cleaning Solutions: Many household cleaning solutions are liquid-liquid mixtures. For instance, window cleaners often contain a mixture of alcohol (isopropyl alcohol or ethanol) and water.
Examples in Industrial Applications:
-
Petroleum Products: Crude oil is a complex mixture of various hydrocarbons (alkanes, alkenes, etc.), all dissolved in each other. The refining process separates these components into various fuels and other useful products.
-
Pharmaceutical Solutions: Many liquid medicines are prepared as solutions of active pharmaceutical ingredients (APIs) dissolved in suitable solvents like water, ethanol, or propylene glycol. These solvents aid in dissolving the API and enhancing its bioavailability.
-
Paints and Coatings: Many paints and coatings consist of pigments and resins dissolved in organic solvents like toluene or xylene. The solvent evaporates after application, leaving behind a solid film.
-
Polymer Solutions: Certain polymers can be dissolved in suitable organic solvents to create solutions used in various applications, such as adhesives, coatings, and textile treatments.
Examples in Nature:
-
Seawater: While primarily a solution of salts in water, seawater also contains dissolved gases (oxygen, carbon dioxide) and organic compounds.
-
Plant Sap: Plant sap is a complex liquid-liquid solution containing water, sugars, amino acids, hormones, and minerals. It plays a vital role in transporting nutrients throughout the plant.
-
Blood Plasma: Blood plasma, the liquid component of blood, is a complex solution containing various dissolved proteins, electrolytes, glucose, and other substances.
-
Cytoplasm: The cytoplasm within cells is a complex liquid-liquid solution containing water, dissolved ions, metabolites, and various other biomolecules.
Factors Affecting Solubility in Liquid-Liquid Solutions
The solubility of one liquid in another is influenced by several factors:
-
Temperature: As mentioned, temperature typically increases solubility, but exceptions exist.
-
Molecular Structure: The shape and size of molecules influence their ability to interact with each other. Similar molecular structures and shapes tend to promote miscibility.
-
Hydrogen Bonding: The presence of hydrogen bonding significantly impacts solubility, particularly in aqueous solutions. Molecules capable of hydrogen bonding readily dissolve in water.
-
Dipole-Dipole Interactions: The interaction between polar molecules increases solubility.
-
London Dispersion Forces: These weak forces are present in all molecules and contribute to solubility, particularly in nonpolar systems.
Applications of Liquid-Liquid Solutions
Liquid-liquid solutions find extensive applications across a broad spectrum:
-
Medicine: As solvents for drug delivery, enhancing absorption and bioavailability.
-
Industry: In various manufacturing processes, as solvents, cleaning agents, and components in products.
-
Cosmetics: Many cosmetic products are liquid-liquid solutions, providing a smooth, even application.
-
Food and Beverage Industry: As flavorings, preservatives, and solvents in food and beverages.
-
Environmental Science: Understanding liquid-liquid solutions is crucial for managing water pollution and wastewater treatment.
Miscibility vs. Immiscibility
It's crucial to distinguish between miscible and immiscible liquids. Miscible liquids are those that completely dissolve in each other in all proportions, forming a homogeneous solution. Examples include ethanol and water. Immiscible liquids do not mix significantly; instead, they form distinct layers. Oil and water are a classic example of immiscible liquids.
Conclusion: The Importance of Liquid-Liquid Solutions
Liquid-liquid solutions are fundamental to numerous aspects of our lives and various scientific disciplines. From the beverages we consume to the medicines we take and the industrial processes that shape our world, understanding the principles governing these solutions is paramount. The examples discussed here represent just a fraction of the vast diversity of liquid-liquid solutions found in nature and created by humans. Further exploration into this topic will reveal the intricate interplay of intermolecular forces, polarity, and other factors that determine the solubility of one liquid within another. This knowledge is not merely theoretical; it's critical for advancements in various fields, from developing new materials to improving environmental sustainability.
Latest Posts
Latest Posts
-
Er And Ir Verbs In Spanish
Mar 18, 2025
-
The Movement Of Water Across A Semipermeable Membrane Is Called
Mar 18, 2025
-
How To Tell If A Reaction Is Spontaneous
Mar 18, 2025
-
Final Electron Acceptor In Aerobic Respiration
Mar 18, 2025
-
Can A Non Polar Molecule Contain Polar Bonds
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Examples Of A Liquid Dissolved In A Liquid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
