How Does Water Have A Higher Boiling Point Than Sulfide
Muz Play
Mar 28, 2025 · 5 min read
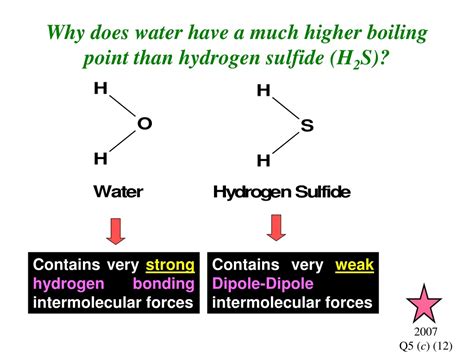
Table of Contents
How Does Water Have a Higher Boiling Point Than Hydrogen Sulfide? A Deep Dive into Intermolecular Forces
Water (H₂O) and hydrogen sulfide (H₂S) are both small molecules with similar structures. Yet, water boils at 100°C, while hydrogen sulfide boils at -60°C. This massive difference in boiling points isn't due to differences in molecular weight; it stems from the vastly different strengths of their intermolecular forces. Understanding this difference requires a deeper exploration of these forces and their impact on the physical properties of substances.
Understanding Boiling Points and Intermolecular Forces
Before diving into the specifics of water and hydrogen sulfide, let's establish a fundamental understanding. A substance's boiling point is the temperature at which its liquid phase transitions to its gaseous phase. This transition requires overcoming the attractive forces holding the molecules together in the liquid state. These forces, collectively known as intermolecular forces, are crucial in determining a substance's boiling point. The stronger the intermolecular forces, the more energy (and thus, higher temperature) is needed to break them and initiate boiling.
Several types of intermolecular forces exist, with varying strengths:
-
London Dispersion Forces (LDFs): Present in all molecules, these forces are caused by temporary fluctuations in electron distribution creating temporary dipoles. These are generally weak, increasing with molecular size and surface area.
-
Dipole-Dipole Forces: Occur in polar molecules (molecules with a permanent dipole moment due to unequal electron sharing). These are stronger than LDFs.
-
Hydrogen Bonding: A special type of dipole-dipole interaction involving a hydrogen atom bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) and another electronegative atom. This is the strongest type of intermolecular force.
The Role of Molecular Structure and Polarity
The key to understanding the boiling point difference between water and hydrogen sulfide lies in their molecular structures and resulting polarities.
Water (H₂O): A Highly Polar Molecule
The water molecule is bent, with a bond angle of approximately 104.5°. Oxygen is significantly more electronegative than hydrogen, resulting in a substantial dipole moment. The oxygen atom carries a partial negative charge (δ-), while the hydrogen atoms carry partial positive charges (δ+). This high polarity leads to strong dipole-dipole interactions and, critically, hydrogen bonding between water molecules. Each water molecule can form up to four hydrogen bonds with neighboring molecules, creating a strong, extensive network.
Hydrogen Sulfide (H₂S): A Less Polar Molecule
Hydrogen sulfide has a bent structure similar to water, but the difference in electronegativity between sulfur and hydrogen is much smaller than that between oxygen and hydrogen. This results in a much weaker dipole moment compared to water. While H₂S exhibits dipole-dipole interactions, they are significantly weaker than the hydrogen bonds in water. Furthermore, sulfur is much less electronegative than oxygen, preventing the formation of strong hydrogen bonds.
Comparing Intermolecular Forces: Water vs. Hydrogen Sulfide
The table below summarizes the key differences in intermolecular forces between water and hydrogen sulfide:
| Feature | Water (H₂O) | Hydrogen Sulfide (H₂S) |
|---|---|---|
| Molecular Polarity | Highly polar | Less polar |
| Hydrogen Bonding | Present (strong) | Absent |
| Dipole-Dipole | Present (strong due to polarity) | Present (weak due to low polarity) |
| London Dispersion Forces | Present (weak) | Present (slightly stronger than in H₂O) |
As you can see, the strength of intermolecular forces is dramatically different. Water's extensive hydrogen bonding network requires significantly more energy to break than the weaker dipole-dipole interactions and London Dispersion Forces in hydrogen sulfide. This explains why water has a much higher boiling point.
The Impact of Molecular Weight: A Minor Player
While molecular weight influences intermolecular forces (primarily LDFs), it's not the primary factor determining the boiling point difference here. Hydrogen sulfide (34 g/mol) has a slightly higher molecular weight than water (18 g/mol), suggesting it should have slightly stronger LDFs. However, the enormous difference in the strength of hydrogen bonding in water completely overshadows this effect. The stronger hydrogen bonds in water far outweigh the marginally stronger LDFs in hydrogen sulfide.
Consequences of the Boiling Point Difference
The significant difference in boiling points has crucial implications for the properties and behavior of both substances:
-
Water's Unique Properties: Water's high boiling point is essential for life on Earth. It allows liquid water to exist over a wide range of temperatures, crucial for biological processes. The strong hydrogen bonding also contributes to water's high surface tension, specific heat capacity, and other properties essential for life.
-
Hydrogen Sulfide's Properties: The low boiling point of hydrogen sulfide means it exists primarily as a gas at room temperature. This has implications for its handling and applications, which are quite different from those of water. It’s a toxic and flammable gas, requiring special safety precautions.
Further Considerations: Other Factors influencing Boiling Point
While intermolecular forces are the dominant factor affecting boiling points, other minor factors can play a role:
-
Molecular Shape: The specific three-dimensional arrangement of atoms can influence the effectiveness of intermolecular interactions.
-
Packing Efficiency: How efficiently molecules pack together in the liquid phase can affect the strength of intermolecular forces.
Conclusion: The Dominant Role of Hydrogen Bonding
In conclusion, the significantly higher boiling point of water compared to hydrogen sulfide is primarily due to the strong hydrogen bonding present in water but absent in hydrogen sulfide. While other intermolecular forces and factors play minor roles, the massive difference in hydrogen bonding strength is the overarching explanation for this dramatic difference in their physical properties. Understanding the influence of intermolecular forces is fundamental to comprehending the behavior of substances and their applications. The unique properties of water, largely stemming from hydrogen bonding, are crucial for the existence and sustenance of life as we know it.
Latest Posts
Latest Posts
-
Why Do Plants Love Water In Bio Terms
Mar 31, 2025
-
Identifying The Important Intermolecular Forces In Pure Compounds
Mar 31, 2025
-
Why Does Km Increase In Competitive Inhibition
Mar 31, 2025
-
What Is The Electron Configuration Of Beryllium
Mar 31, 2025
-
When Do You Consider Log Diterminants Similar
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about How Does Water Have A Higher Boiling Point Than Sulfide . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
