How Many Bonds Can Aluminum Form
Muz Play
Mar 18, 2025 · 5 min read
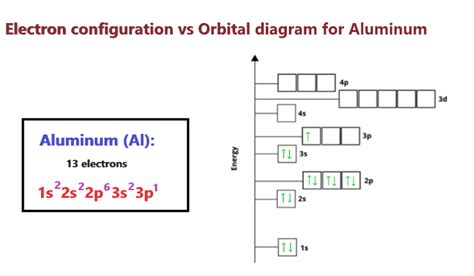
Table of Contents
How Many Bonds Can Aluminum Form? Delving into the Bonding Capabilities of Aluminum
Aluminum, a ubiquitous element found in everything from soda cans to aircraft, boasts a fascinating chemistry characterized by its unique bonding capabilities. Understanding how many bonds aluminum can form is crucial to grasping its diverse applications and its behavior in various chemical contexts. While a simple answer might seem straightforward, the reality is more nuanced, encompassing various factors influencing its bonding behavior. This comprehensive article will explore the intricacies of aluminum bonding, unraveling the factors that determine its bond number and highlighting its impact across different chemical scenarios.
The Basics: Aluminum's Electronic Configuration and Bonding Preferences
Aluminum (Al), with an atomic number of 13, possesses the electronic configuration [Ne] 3s²3p¹. This configuration dictates its bonding preferences. The three valence electrons (those in the outermost shell) are readily available for participation in chemical bonds. This directly points to a maximum potential of three bonds for aluminum. However, this is a simplified picture, and the actual number of bonds formed often deviates from this ideal due to several factors.
The Octet Rule and Its Implications for Aluminum
The octet rule, while not absolute, serves as a useful guideline. It suggests that atoms tend to gain, lose, or share electrons to achieve a stable electron configuration resembling that of a noble gas, typically with eight electrons in their valence shell. For aluminum, achieving an octet means either losing three electrons (forming Al³⁺ cations) or sharing three electrons to form covalent bonds.
Factors Influencing the Number of Bonds Formed by Aluminum
Several factors intricately influence the actual number of bonds formed by aluminum:
1. Electronegativity and Bond Polarity:
Aluminum's electronegativity is relatively low (1.61 on the Pauling scale). This means it's less likely to attract electrons strongly compared to more electronegative elements. Consequently, in bonds with significantly more electronegative elements like oxygen (O) or chlorine (Cl), aluminum will often form predominantly ionic bonds, losing its three valence electrons to become Al³⁺. However, in bonds with elements of similar electronegativity, such as other metals, metallic bonding dominates.
2. Steric Hindrance:
The size of the surrounding atoms and molecules plays a crucial role. Bulky ligands or neighboring atoms can hinder the approach of additional bonding partners, thereby limiting the number of bonds aluminum can form. This steric hindrance effect is particularly prominent in coordination complexes and organometallic compounds.
3. Coordination Number:
Coordination number refers to the number of atoms or ions directly bonded to a central atom. In aluminum's case, its coordination number is often influenced by factors such as the size and charge of the ligands and the geometry of the resulting complex. While three is common, higher coordination numbers, such as four, five, or even six, are observed in various aluminum compounds.
Exploring Aluminum's Bonding in Different Chemical Contexts:
Let's examine aluminum's bonding in specific chemical contexts:
1. Aluminum Halides (AlX₃):
Aluminum halides (AlF₃, AlCl₃, AlBr₃, AlI₃) represent a classic example. Their behavior deviates from the simple expectation of three bonds. While the simplest depiction shows aluminum with three bonds, the actual structure and bonding are often more complex. For example, AlCl₃ exists as a dimer (Al₂Cl₆) in the solid state and non-polar solvents, featuring four bonds per aluminum atom. In the gas phase, monomeric AlCl₃ with three bonds is observed. The dimerization is driven by the enhanced stability achieved through achieving a complete octet for aluminum.
2. Aluminum Oxides (Al₂O₃):
Aluminum oxide (Al₂O₃), also known as alumina, is a crucial compound with a complex structure. Aluminum atoms in alumina are surrounded by four or six oxygen atoms, exhibiting coordination numbers of four and six, respectively. This demonstrates that the simple three-bond model is inadequate for describing the bonding in this extensively studied and technologically significant compound.
3. Aluminum Organometallic Compounds:
In organometallic chemistry, aluminum forms bonds with carbon-containing groups. These compounds display a diverse range of bonding scenarios, often exceeding three bonds per aluminum atom. For instance, many aluminum alkyls exhibit bridging alkyl groups, leading to coordination numbers exceeding three. Steric factors and the nature of the organic ligands significantly influence the coordination number in these systems.
4. Aluminum Hydrides (AlH₃):
Aluminum hydride (AlH₃) exists in various polymeric forms. The bonding is complex and involves bridging hydrogen atoms, resulting in a network structure where the number of bonds around an aluminum atom isn't simply three. Understanding the hydrogen bridging and overall polymeric structure is crucial to comprehending the bonding in aluminum hydrides.
Beyond Simple Counting: Understanding Bond Types
It's essential to differentiate between different types of bonds when discussing aluminum's bonding. The simple "number of bonds" overlooks the crucial aspects of:
- Ionic bonds: Aluminum readily loses three electrons to form the Al³⁺ cation, characteristic of ionic bonding with electronegative elements.
- Covalent bonds: Aluminum can share electrons with other atoms, forming covalent bonds, particularly with less electronegative elements or in molecules where charge distribution is more uniform.
- Metallic bonds: In metallic aluminum and aluminum alloys, metallic bonding dominates, involving a sea of delocalized electrons.
The relative contributions of these bond types determine the overall properties of the aluminum-containing material.
Conclusion: The Versatile Bonding of Aluminum
In conclusion, while aluminum possesses three valence electrons and thus theoretically could form three bonds, the reality is significantly more nuanced. The number of bonds formed depends on multiple factors, including electronegativity differences, steric effects, coordination number, and the overall chemical environment. Aluminum exhibits diverse bonding characteristics, ranging from predominantly ionic to covalent and metallic, leading to its wide-ranging applications in various fields. Instead of focusing solely on a fixed number, understanding the interplay of these factors provides a much more comprehensive picture of aluminum's fascinating and versatile bonding behavior. This detailed understanding is crucial for the continued development of aluminum-based materials and technologies.
Latest Posts
Latest Posts
-
Is A Solution A Homogeneous Or Heterogeneous Mixture
Mar 18, 2025
-
Is S More Electronegative Than O
Mar 18, 2025
-
How Many Valence Electrons Are In Sulfur
Mar 18, 2025
-
What Is The Difference Between Volume Measurements And Capacities
Mar 18, 2025
-
In A Polar Covalent Bond Electrons Are Shared
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about How Many Bonds Can Aluminum Form . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
