How Many Oxygen Atoms Are In H20
Muz Play
Mar 28, 2025 · 6 min read
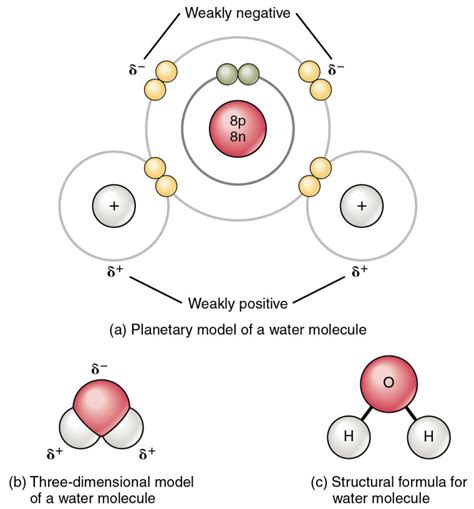
Table of Contents
How Many Oxygen Atoms Are in H₂O? A Deep Dive into Water's Composition
Water, the elixir of life, is a deceptively simple molecule with a profound impact on our world. Its chemical formula, H₂O, is familiar to most, but understanding the very essence of this formula – the number of atoms involved – unlocks a deeper appreciation for its properties and significance. So, how many oxygen atoms are in H₂O? The answer, quite simply, is one.
Deconstructing the H₂O Formula
The chemical formula H₂O provides a concise description of a water molecule's composition. Let's break it down:
- H: This symbol represents the element hydrogen. Each "H" signifies a single hydrogen atom.
- ₂: This subscript indicates that there are two hydrogen atoms present in each water molecule.
- O: This symbol represents the element oxygen. It signifies a single oxygen atom.
Therefore, the formula H₂O tells us that each water molecule contains two hydrogen atoms and one oxygen atom. This fundamental understanding is crucial for grasping many of water's unique properties.
The Importance of Atomic Structure
To truly appreciate the presence of that single oxygen atom in H₂O, we need to consider the atomic structure of both hydrogen and oxygen.
Oxygen's Atomic Structure
Oxygen (O) has an atomic number of 8, meaning it has 8 protons in its nucleus. To achieve stability, it needs to fill its outer electron shell, which requires 8 electrons. Oxygen has 6 electrons in its outer shell, meaning it needs to gain 2 more electrons to achieve a stable octet. This tendency to gain electrons makes oxygen highly reactive and crucial for many chemical processes.
Hydrogen's Atomic Structure
Hydrogen (H) has an atomic number of 1, possessing one proton and one electron. Its outer electron shell only needs two electrons to be full. Hydrogen readily shares its electron or gains an electron to achieve this stable configuration.
Covalent Bonding in H₂O
The formation of a water molecule involves covalent bonding. This type of bond occurs when atoms share electrons to achieve a stable electron configuration. In H₂O:
- The oxygen atom shares one electron with each of the two hydrogen atoms.
- Each hydrogen atom shares its single electron with the oxygen atom.
This sharing results in the oxygen atom having a complete octet (8 electrons in its outer shell) and each hydrogen atom having a full outer shell (2 electrons). This stable arrangement forms the robust and relatively unreactive water molecule.
The Unique Properties of Water Stemming from its Composition
The presence of one oxygen atom and two hydrogen atoms in H₂O is not merely a matter of counting. This specific arrangement and the resulting covalent bonds give water its unique properties, essential for life as we know it. These properties include:
1. Polarity and Hydrogen Bonding
The oxygen atom in H₂O is more electronegative than the hydrogen atoms. This means it attracts the shared electrons more strongly, creating a slightly negative charge (δ-) near the oxygen atom and slightly positive charges (δ+) near the hydrogen atoms. This uneven distribution of charge gives water a polar nature. The positive end of one water molecule is attracted to the negative end of another, forming hydrogen bonds. These weak bonds are responsible for many of water's exceptional properties.
2. High Specific Heat Capacity
Water has an unusually high specific heat capacity, meaning it can absorb a large amount of heat with only a small increase in temperature. This property is crucial for regulating temperature, both in the environment and within living organisms. The extensive hydrogen bonding network within water requires significant energy to break, making it resistant to temperature changes. The single oxygen atom at the heart of each molecule contributes significantly to this network.
3. High Heat of Vaporization
Water has a high heat of vaporization, meaning it takes a considerable amount of energy to convert liquid water into water vapor. This property is essential for cooling processes, such as sweating in humans and transpiration in plants. Again, the hydrogen bonds, facilitated by the central oxygen atom, are responsible for this characteristic.
4. Universal Solvent
Water's polarity makes it an excellent solvent, meaning it can dissolve many ionic and polar substances. The slightly positive hydrogen atoms and the slightly negative oxygen atom can interact with and surround charged particles, effectively dissolving them. This property is crucial for many biological processes, as water acts as a medium for transporting nutrients and carrying out metabolic reactions. The single oxygen atom plays a critical role in this solubility.
5. Density Anomaly
Ice is less dense than liquid water, a unique property that allows ice to float. This is due to the hydrogen bonding network forming a crystalline structure in ice that is less compact than the liquid phase. The oxygen atom is pivotal in the formation of this network.
Beyond the Single Oxygen Atom: Isotopes and Water Variations
While the standard water molecule contains one oxygen-16 atom (¹⁶O), other isotopes of oxygen exist, such as oxygen-17 (¹⁷O) and oxygen-18 (¹⁸O). These isotopes have the same number of protons but a different number of neutrons. The presence of these isotopes leads to variations in water's properties, though these differences are subtle.
The abundance of each oxygen isotope in water varies slightly depending on the source and environmental factors. The study of these isotopic variations is used in various scientific fields, including hydrology, paleoclimatology, and geochemistry.
Applications and Significance of Understanding H₂O's Composition
Understanding the composition of water, particularly the crucial role of the single oxygen atom, has far-reaching implications across diverse fields:
- Biology: The properties of water are fundamental to all life processes. The transport of nutrients, metabolic reactions, and temperature regulation all rely on water's unique characteristics, directly related to its molecular structure.
- Chemistry: Water serves as a reactant or solvent in countless chemical reactions. Its polar nature and ability to form hydrogen bonds make it a crucial component in many chemical processes.
- Environmental Science: Understanding the distribution and behavior of water is crucial for managing water resources, predicting weather patterns, and studying climate change. Isotopic analysis of water provides valuable insights into these processes.
- Medicine: Water is essential for human health, maintaining proper hydration, and facilitating various bodily functions.
- Industry: Water is used extensively in various industries, from agriculture to manufacturing. Understanding its properties is crucial for optimizing processes and managing resources.
Conclusion: The Unsung Hero – The Single Oxygen Atom in H₂O
The seemingly simple formula H₂O belies the complexity and importance of the water molecule. While the presence of two hydrogen atoms is significant, it's the single oxygen atom at the heart of the molecule that dictates its extraordinary properties. This single atom facilitates the formation of hydrogen bonds, a cornerstone of water's unique characteristics. Understanding the number and role of each atom within the H₂O molecule provides a deeper appreciation for this vital substance and its profound influence on our planet and all life upon it. From the smallest cell to the largest ocean, the single oxygen atom in H₂O is the unsung hero of life itself.
Latest Posts
Latest Posts
-
What Makes Something A Strong Acid
Mar 31, 2025
-
According To The Rules Of Osmosis A System Will
Mar 31, 2025
-
Where Are Chondrocytes And Osteocytes Located
Mar 31, 2025
-
List The Types Of Persuasive Speeches
Mar 31, 2025
-
The Energy Needed To Start A Chemical Reaction Is Called
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about How Many Oxygen Atoms Are In H20 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
