How Many Valence Electrons Does Cobalt Have
Muz Play
Mar 17, 2025 · 6 min read
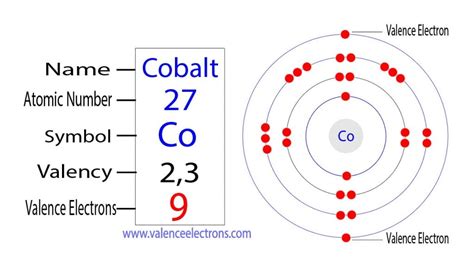
Table of Contents
How Many Valence Electrons Does Cobalt Have? A Deep Dive into Electronic Configuration and Chemical Behavior
Cobalt, a transition metal with the symbol Co and atomic number 27, plays a crucial role in various applications, from industrial catalysts to biomedical devices. Understanding its electronic structure, particularly the number of valence electrons, is key to comprehending its chemical behavior and reactivity. This article delves deep into the electronic configuration of cobalt, explaining why it possesses the number of valence electrons it does and how this influences its diverse chemical properties.
Understanding Valence Electrons
Before we delve into the specifics of cobalt, let's establish a clear understanding of what valence electrons are. Valence electrons are the electrons located in the outermost shell (principal energy level) of an atom. These electrons are most loosely bound to the nucleus and are primarily responsible for an atom's chemical behavior, participating in the formation of chemical bonds with other atoms. The number of valence electrons dictates an element's reactivity and the types of bonds it can form (ionic, covalent, metallic).
Cobalt's Electronic Configuration: The Key to Understanding Valence Electrons
To determine the number of valence electrons in cobalt, we need to examine its electron configuration. The electron configuration describes how electrons are distributed among the various energy levels and sublevels within an atom. For cobalt (atomic number 27), the complete electron configuration is:
1s²2s²2p⁶3s²3p⁶4s²3d⁷
This configuration indicates the following distribution of electrons:
- 1s²: Two electrons in the first energy level (1s sublevel)
- 2s²2p⁶: Eight electrons in the second energy level (2s and 2p sublevels)
- 3s²3p⁶: Eight electrons in the third energy level (3s and 3p sublevels)
- 4s²3d⁷: Nine electrons in the fourth energy level (4s and 3d sublevels). Note the filling order—the 4s sublevel fills before the 3d sublevel.
Now, to pinpoint the valence electrons, we focus on the outermost shell. While the 4s electrons are undeniably in the outermost shell, the involvement of 3d electrons in chemical bonding adds a layer of complexity specific to transition metals.
The Complexity of Valence Electrons in Transition Metals
Unlike main group elements where valence electrons are clearly defined by the outermost shell, transition metals like cobalt exhibit a more nuanced behavior. Their d electrons, although not strictly in the outermost shell, actively participate in chemical bonding, effectively behaving as valence electrons. This is because the energy difference between the outermost s and d orbitals is relatively small, allowing for the participation of both in chemical reactions.
How Many Valence Electrons Does Cobalt Possess?
Considering the active participation of both 4s and 3d electrons in cobalt's chemical bonding, we conclude that cobalt has nine valence electrons. This means it has two electrons in the 4s orbital and seven electrons in the 3d orbital. This unique electron configuration is responsible for cobalt's diverse chemical properties and ability to form a wide range of compounds with varying oxidation states.
Cobalt's Variable Oxidation States: A Consequence of its Valence Electrons
The presence of nine valence electrons allows cobalt to exhibit multiple oxidation states. This means that cobalt atoms can lose a varying number of electrons to form ions with different charges. Common oxidation states for cobalt include +2 (Co²⁺) and +3 (Co³⁺), but others, such as +1, +4, and even higher oxidation states, can exist under specific conditions.
The ability to exhibit variable oxidation states stems directly from the availability of both 4s and 3d electrons for participation in bonding. Depending on the chemical environment and the reacting species, cobalt can lose electrons from both the 4s and 3d orbitals, leading to different oxidation states.
The Influence of Valence Electrons on Cobalt's Chemical Properties
The nine valence electrons directly influence cobalt's chemical properties in several ways:
-
Complex Formation: Cobalt's ability to form coordination complexes is remarkable. The d electrons in the 3d orbitals readily participate in forming coordinate covalent bonds with ligands (molecules or ions that donate electron pairs). This leads to the formation of a wide array of colorful and structurally diverse complexes, with applications in various fields, including catalysis and medicine.
-
Catalytic Activity: Cobalt's variable oxidation states and ability to form complexes make it a highly effective catalyst in numerous industrial processes. It can easily switch between different oxidation states during a catalytic cycle, facilitating chemical reactions. This is crucial in applications like Fischer-Tropsch synthesis (production of hydrocarbons from synthesis gas) and various oxidation reactions.
-
Magnetic Properties: The partially filled 3d orbitals give cobalt unique magnetic properties. It exhibits ferromagnetism, meaning it can retain a magnetic field even after the external magnetic field is removed. This property is essential in the production of powerful permanent magnets used in various technologies.
-
Reactivity: Cobalt's reactivity is influenced by its readily available valence electrons. It reacts with various elements and compounds, forming oxides, sulfides, halides, and other compounds. The specific reaction pathways are dependent on the oxidation state of cobalt and the reacting species.
Applications Leveraging Cobalt's Valence Electrons
The unique properties stemming from cobalt's nine valence electrons have led to widespread applications across numerous fields:
-
Catalysis: Cobalt catalysts are used in various industrial processes, such as hydroformylation, oxidation reactions, and Fischer-Tropsch synthesis. The ability to readily change oxidation states is pivotal for its catalytic activity.
-
Magnets: Cobalt-based alloys are used in the production of high-performance permanent magnets found in applications like electric motors, generators, and magnetic resonance imaging (MRI) machines.
-
Alloys: Cobalt is added to various alloys to improve their hardness, strength, and corrosion resistance. These alloys find applications in high-temperature tools, aerospace components, and medical implants.
-
Superalloys: Cobalt-based superalloys exhibit exceptional high-temperature strength and corrosion resistance, making them essential components in gas turbines and jet engines.
-
Biomedical Applications: Cobalt compounds have shown promise in various biomedical applications, including cancer therapy and as components of orthopedic implants.
Conclusion: Cobalt's Valence Electrons – A Foundation for its Versatility
The number of valence electrons an atom possesses is fundamental to understanding its chemical behavior. Cobalt, with its nine valence electrons (two 4s and seven 3d), exemplifies the importance of considering both the outermost shell and the participation of d electrons in the chemical properties of transition metals. This unique electronic configuration is directly responsible for cobalt's variable oxidation states, its exceptional ability to form complexes, its catalytic activity, and its diverse magnetic properties. These properties, in turn, underpin its numerous applications across various fields, showcasing the vital role of valence electrons in determining an element's utility and importance in science and technology. The continued investigation and understanding of cobalt's electronic structure will undoubtedly lead to even further advancements and innovations in the future.
Latest Posts
Latest Posts
-
What Are The Three Basic Components Of An Atom
Mar 17, 2025
-
Does Gas Have A Definite Shape
Mar 17, 2025
-
If The Equilibrium Constant Is Negative What Does That Mean
Mar 17, 2025
-
How Does An Atom Become A Cation
Mar 17, 2025
-
Which Body Cavity Protects The Spinal Column
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Does Cobalt Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
