How To Name Acids And Bases
Muz Play
Mar 24, 2025 · 5 min read
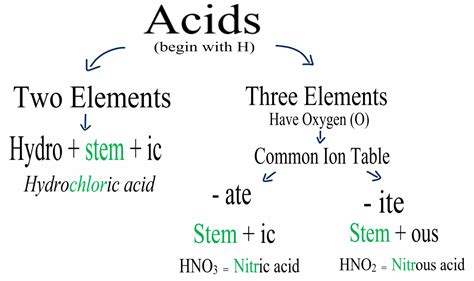
Table of Contents
How to Name Acids and Bases: A Comprehensive Guide
Naming chemical compounds might seem daunting, but with a systematic approach, it becomes manageable. This comprehensive guide will equip you with the knowledge to confidently name acids and bases, two fundamental classes of chemical compounds. We'll cover the nomenclature rules, provide examples, and offer tips to solidify your understanding.
Understanding Acids and Bases
Before diving into nomenclature, let's briefly revisit the definitions of acids and bases. Several definitions exist, but the most common are the Arrhenius and Brønsted-Lowry definitions.
-
Arrhenius Definition: An acid is a substance that produces hydrogen ions (H⁺) when dissolved in water, while a base produces hydroxide ions (OH⁻). This definition is limited to aqueous solutions.
-
Brønsted-Lowry Definition: A broader definition, this considers acids as proton (H⁺) donors and bases as proton acceptors. This definition encompasses reactions that don't necessarily involve water.
This guide will primarily focus on naming acids and bases based on their chemical formulas, aligning mostly with the Arrhenius definition for simplicity in naming conventions.
Naming Acids
Acid nomenclature depends on whether the acid is derived from a binary compound (containing only two elements) or an oxyacid (containing oxygen, hydrogen, and another element).
Naming Binary Acids
Binary acids are composed of hydrogen and a nonmetal. Their names follow a simple pattern:
- Prefix: The prefix "hydro-" is always used.
- Stem: The stem of the nonmetal's name is used.
- Suffix: The suffix "-ic" is added, followed by the word "acid".
Examples:
- HCl: Hydrochloric acid (hydrogen + chlorine)
- HBr: Hydrobromic acid (hydrogen + bromine)
- HI: Hydroiodic acid (hydrogen + iodine)
- H₂S: Hydrosulfuric acid (hydrogen + sulfur) Note the use of "sulfur" instead of "sulfur" here. This is a commonly seen exception.
- HF: Hydrofluoric acid (hydrogen + fluorine)
Naming Oxyacids
Oxyacids contain hydrogen, oxygen, and a nonmetal (or sometimes a metalloid). Their naming is a bit more complex and involves considering the oxidation state of the central nonmetal:
-
Identify the anion: First, identify the anion (negatively charged ion) formed by the nonmetal and oxygen. This anion will have a suffix that indicates the oxidation state of the central nonmetal.
-
Suffixes:
- -ate: If the anion has the higher oxidation state of the nonmetal, the suffix "-ate" is used.
- -ite: If the anion has the lower oxidation state of the nonmetal, the suffix "-ite" is used.
-
Acid Name: Replace the "-ate" suffix with "-ic acid" and the "-ite" suffix with "-ous acid".
Examples:
Let's consider the oxyacids of sulfur:
- H₂SO₄ (Sulfate ion, SO₄²⁻): Sulfuric acid (higher oxidation state of sulfur)
- H₂SO₃ (Sulfite ion, SO₃²⁻): Sulfurous acid (lower oxidation state of sulfur)
Now let's look at the oxyacids of phosphorus:
- H₃PO₄ (Phosphate ion, PO₄³⁻): Phosphoric acid
- H₃PO₃ (Phosphite ion, PO₃³⁻): Phosphorous acid
Special Cases:
Some oxyacids have more than two oxidation states. In these instances, prefixes like "hypo-" (for the lowest oxidation state) and "per-" (for the highest oxidation state) are used.
- HClO₄ (Perchlorate ion, ClO₄⁻): Perchloric acid
- HClO₃ (Chlorate ion, ClO₃⁻): Chloric acid
- HClO₂ (Chlorite ion, ClO₂⁻): Chlorous acid
- HClO (Hypochlorite ion, ClO⁻): Hypochlorous acid
Naming Bases
Naming bases is generally simpler than naming acids. Many common bases are metal hydroxides. Their names follow this pattern:
-
Cation Name: The name of the metal cation (positively charged ion) is written first. If the metal has multiple oxidation states (like iron or copper), the oxidation state is indicated using Roman numerals in parentheses.
-
Hydroxide Ion: The word "hydroxide" is added.
Examples:
- NaOH: Sodium hydroxide (Sodium is a group 1 metal, thus it only has one oxidation state)
- KOH: Potassium hydroxide
- Ca(OH)₂: Calcium hydroxide
- Fe(OH)₂: Iron(II) hydroxide
- Fe(OH)₃: Iron(III) hydroxide
- Cu(OH)₂: Copper(II) hydroxide
- CuOH: Copper(I) hydroxide
Tips and Tricks for Mastering Acid and Base Nomenclature
-
Practice Regularly: Consistent practice is key to mastering nomenclature. Work through numerous examples, focusing on identifying the cation and anion, and then applying the appropriate rules.
-
Memorize Common Ions: Familiarity with common polyatomic ions (like sulfate, phosphate, nitrate, etc.) will greatly simplify the process. Create flashcards or use other memorization techniques.
-
Use a Periodic Table: A periodic table can be invaluable in determining the oxidation state of elements and identifying whether an element is a metal or nonmetal.
-
Understand Oxidation States: A solid grasp of oxidation states is crucial, especially for naming oxyacids. Practice calculating oxidation states for different elements and compounds.
-
Consult a Reference Book: When unsure, always consult a reputable chemistry textbook or online resource for clarification.
-
Work Backwards: A helpful exercise is to start with the name of an acid or base and try to deduce its chemical formula. This reinforces your understanding of the naming conventions.
Advanced Naming Considerations
Beyond the basic rules outlined above, there are some advanced scenarios you might encounter:
-
Organic Acids: Organic acids, containing carbon, hydrogen, and oxygen (often with other elements), follow different naming conventions. These often involve common names and IUPAC nomenclature, which is a more complex system beyond the scope of this basic guide.
-
Complex Ions: When dealing with acids or bases containing complex ions (ions composed of multiple atoms bonded together), the naming process becomes more involved, often requiring knowledge of coordination chemistry.
-
Hydrates: Some acids and bases can exist as hydrates – meaning they incorporate water molecules into their crystal structure. The name of the hydrate includes a prefix indicating the number of water molecules per formula unit (e.g., copper(II) sulfate pentahydrate).
Conclusion
Naming acids and bases is a fundamental skill in chemistry. By understanding the rules outlined in this guide and practicing consistently, you can confidently name a wide range of acids and bases. Remember that mastering nomenclature is a gradual process, so don't be discouraged by initial challenges. With dedication and consistent effort, you will become proficient in this crucial aspect of chemical communication. Continue to explore more advanced topics as your knowledge grows, delving into organic acids, complex ions, and the nuances of IUPAC nomenclature. The world of chemistry is vast and rewarding!
Latest Posts
Latest Posts
-
How Do You Calculate Index Numbers
Mar 26, 2025
-
What Type Of Bonding Involves The Unequal Sharing Of Electrons
Mar 26, 2025
-
What Is A Calorie In Chemistry
Mar 26, 2025
-
Gramatica A The Verb Gustar Worksheet Answers
Mar 26, 2025
-
What Is The Vertical Columns On The Periodic Table Called
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about How To Name Acids And Bases . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
