Identify The Most Acidic Proton In The Compound
Muz Play
Mar 28, 2025 · 6 min read
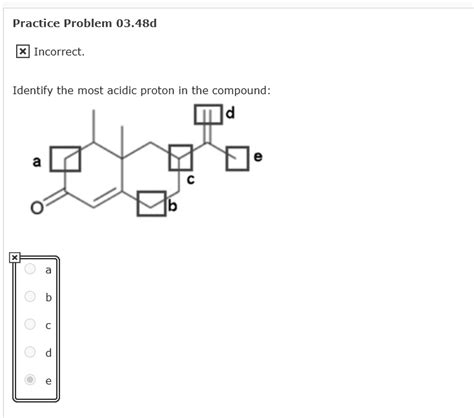
Table of Contents
Identifying the Most Acidic Proton in a Compound: A Comprehensive Guide
Determining the most acidic proton in a molecule is a fundamental concept in organic chemistry with significant implications for reaction mechanisms, synthesis strategies, and understanding molecular behavior. This seemingly simple task requires a thorough understanding of several key factors, including the stability of the resulting conjugate base, inductive effects, resonance stabilization, and hybridization. This article will delve into these concepts, providing a comprehensive guide to identifying the most acidic proton in a variety of compounds.
Understanding Acidity: The Role of the Conjugate Base
Acidity is defined by the willingness of a molecule to donate a proton (H⁺). The stronger the acid, the more readily it donates its proton. This propensity is directly related to the stability of the resulting conjugate base. The more stable the conjugate base, the stronger the acid. This is the cornerstone of understanding acidity. When a proton is removed, the negative charge left behind must be effectively accommodated by the molecule. Several factors influence the stability of this negative charge and thus, the acidity of the original compound.
1. Inductive Effects
Inductive effects refer to the polarization of electron density within a molecule due to the electronegativity difference between atoms. Electronegative atoms, such as halogens (F, Cl, Br, I), oxygen, and nitrogen, draw electron density towards themselves. This effect can significantly stabilize a negative charge on a nearby atom. Consider the following example:
- CH₃CH₂OH vs. CF₃CH₂OH: In trifluoroethanol (CF₃CH₂OH), the highly electronegative fluorine atoms withdraw electron density from the carbon atom adjacent to the hydroxyl group (-OH). This electron withdrawal stabilizes the negative charge on the oxygen atom in the conjugate base, making trifluoroethanol a significantly stronger acid than ethanol (CH₃CH₂OH).
The strength of the inductive effect decreases with distance. The further an electronegative atom is from the acidic proton, the less pronounced its effect on acidity.
2. Resonance Stabilization
Resonance occurs when a negative charge can be delocalized over multiple atoms through pi-bonding. This delocalization significantly increases the stability of the conjugate base. The more resonance structures possible, the greater the stabilization.
Let's compare the acidity of acetic acid (CH₃COOH) and ethanol (CH₃CH₂OH). When the proton from the carboxyl group (-COOH) of acetic acid is removed, the resulting negative charge can be delocalized across the two oxygen atoms through resonance. This resonance stabilization is absent in the ethoxide ion, the conjugate base of ethanol. Therefore, acetic acid is a significantly stronger acid than ethanol.
This principle extends to other functional groups capable of resonance stabilization, such as phenols and carboxylic acids.
3. Hybridization
The hybridization of the atom bearing the acidic proton also influences acidity. The more s-character an orbital has, the closer the electrons are to the nucleus, leading to greater stability. For example, sp-hybridized carbons have 50% s-character, sp²-hybridized carbons have 33% s-character, and sp³-hybridized carbons have 25% s-character.
Consequently, sp-hybridized carbons are more electronegative than sp² and sp³-hybridized carbons, making the proton attached to an sp-hybridized carbon more acidic. This is why terminal alkynes are more acidic than alkenes, which are more acidic than alkanes.
4. Aromaticity
Aromaticity is a special type of resonance stabilization that significantly enhances acidity. Aromatic compounds, such as phenol and pyrrole, exhibit exceptional stability due to the delocalization of electrons within the pi system. Removing a proton from phenol leads to the phenoxide ion, where the negative charge is delocalized throughout the aromatic ring. This resonance stabilization makes phenol significantly more acidic than cyclohexanol.
Similarly, the acidic proton in pyrrole is readily abstracted due to the resulting negative charge being delocalized within the aromatic ring.
Identifying the Most Acidic Proton: A Step-by-Step Approach
Applying the principles discussed above, let's develop a systematic approach to identifying the most acidic proton in a given molecule:
-
Identify all potential acidic protons: These are usually protons attached to electronegative atoms like oxygen, nitrogen, sulfur, or to carbon atoms adjacent to electronegative groups.
-
Analyze the inductive effects: Determine if any electronegative atoms or groups are present nearby. The closer and the more electronegative the atom, the stronger the inductive effect, and therefore, the greater the acidity.
-
Assess resonance stabilization: Evaluate if the negative charge resulting from proton removal can be delocalized through resonance. The greater the extent of resonance, the more stable the conjugate base and the stronger the acid.
-
Consider hybridization: If the acidic proton is on a carbon atom, determine its hybridization (sp, sp², or sp³). Sp-hybridized carbons are the most acidic, followed by sp², and then sp³.
-
Evaluate aromaticity: Determine if the conjugate base will be aromatic. If so, the acidity will be significantly enhanced.
-
Compare the stability of the conjugate bases: After analyzing the inductive effects, resonance, hybridization, and aromaticity, compare the stability of the conjugate bases formed by removing each potential proton. The most stable conjugate base corresponds to the strongest acid and therefore, the most acidic proton.
Examples: Illustrating the Principles
Let's apply this approach to a few examples:
Example 1: Comparing Acidity in a Molecule with Multiple Functional Groups
Consider the molecule containing a carboxylic acid, a phenol, and an alcohol group.
O OH OH
|| | |
CH₃-C-OH -C₆H₄- -CH₂-CH₃
-
Potential acidic protons: The carboxylic acid (-COOH), phenol (-OH), and alcohol (-OH) groups all possess acidic protons.
-
Inductive effects: The carboxylic acid experiences a stronger inductive effect due to the presence of two oxygen atoms.
-
Resonance stabilization: Both the carboxylate ion (from the carboxylic acid) and the phenoxide ion (from phenol) are stabilized by resonance, with the carboxylate ion having more extensive resonance stabilization.
-
Hybridization: All acidic protons are on sp³ hybridized carbons, so hybridization is not a significant differentiating factor here.
-
Aromaticity: The phenoxide ion benefits from aromaticity, further increasing its stability.
-
Conclusion: The carboxylic acid proton is the most acidic due to the combined effect of the strongest inductive effect and significant resonance stabilization. The phenol proton is the second most acidic due to resonance and aromaticity, and the alcohol proton is the least acidic due to lack of significant resonance or inductive effects.
Example 2: A more complex molecule
Let's consider a molecule with a ketone, an amine, and a terminal alkyne.
O
||
CH₃-C-CH₂-CH₂-NH₂ -C≡CH
-
Potential acidic protons: The amine (-NH₂), and the terminal alkyne (-C≡CH) protons are potential acidic protons.
-
Inductive effects: The ketone group exerts a weak inductive effect.
-
Resonance stabilization: The conjugate base of the terminal alkyne is stabilized by resonance within the carbon-carbon triple bond.
-
Hybridization: The terminal alkyne proton is on an sp hybridized carbon, while the amine protons are on an sp³ hybridized nitrogen.
-
Aromaticity: No aromatic stabilization is present.
-
Conclusion: The terminal alkyne proton is the most acidic due to the sp hybridization and the slight resonance stabilization in the conjugate base. The amine proton is the second most acidic, as it is quite easily removed, although less so than the alkyne. The ketone protons are least acidic.
Conclusion
Identifying the most acidic proton in a molecule requires a careful and systematic approach. By thoroughly considering the factors governing conjugate base stability – inductive effects, resonance, hybridization, and aromaticity – one can effectively predict the most acidic proton within a compound. This understanding is crucial for predicting reaction pathways, designing synthesis strategies, and interpreting the properties of organic molecules. Understanding these principles is a fundamental skill for any student or researcher working in the field of organic chemistry.
Latest Posts
Latest Posts
-
What Happens When You Combine An Acid And A Base
Mar 31, 2025
-
How To Solve A Rational Exponent
Mar 31, 2025
-
Heat Of Combustion Of Benzoic Acid
Mar 31, 2025
-
How To Round Sig Figs When Adding
Mar 31, 2025
-
Do Acids Or Bases React With Metals
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Identify The Most Acidic Proton In The Compound . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
